Back to: Organic Chemistry 200 Level
Welcome to class!
Hello brilliant learner! It’s great to have you here again. You’ve already learnt the structures, properties, and key reactions of aldehydes and ketones. Today, we’re focusing on important derivatives and applications of aldehydes and ketones. By the end of this lesson, you’ll see how these compounds go from textbook diagrams to real-life products in industries, laboratories, and even your home.
Aldehydes And Ketones III
Formation of Important Derivatives
Aldehydes and ketones can react with other chemicals to form derivatives — new compounds that are often more stable or easier to identify.
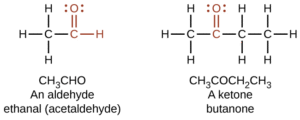
2,4-Dinitrophenylhydrazone (2,4-DNP) Derivatives
Reaction with 2,4-dinitrophenylhydrazine produces bright yellow or orange solids.
These derivatives are useful for confirming the presence of a carbonyl group.
Oximes
Formed when aldehydes or ketones react with hydroxylamine (NH₂OH).
Oximes are often more stable and are used in analytical chemistry.
Hydrazones
Produced when reacting with hydrazine (NH₂NH₂).
Useful for identifying and separating carbonyl compounds.
Bisulphite Addition Compounds
Sodium bisulphite (NaHSO₃) reacts with aldehydes and some ketones to form crystalline solids.
This method can purify aldehydes and ketones from mixtures.
Condensation Reactions
Aldol Condensation
In the presence of a base, aldehydes and ketones with α-hydrogen atoms can join together to form larger molecules (aldols).
This is an important reaction in organic synthesis and industrial chemistry.
Industrial and Everyday Applications
Formaldehyde (Methanal): Used in producing plastics, disinfectants, and in preserving biological specimens.
Acetone (Propanone): Used as a solvent in paints, nail polish removers, and cleaning products.
Flavour and Fragrance Industry: Aldehydes like benzaldehyde give almond flavour; vanillin gives the aroma of vanilla.
Pharmaceuticals: Many drugs contain aldehyde or ketone functional groups as part of their active structure.

Health and Safety Notes
While aldehydes and ketones are very useful, some (like formaldehyde) can be toxic if inhaled or touched. Always handle them with care in well-ventilated areas, using protective gloves and eyewear.
Summary
- Aldehydes and ketones form derivatives like 2,4-DNP, oximes, hydrazones, and bisulphite addition compounds.
- Aldol condensation is a key reaction for building larger molecules.
- They are used in plastics, solvents, perfumes, flavourings, and medicines.
- Safety is important when handling these chemicals.
Evaluation
- Name two derivatives of aldehydes or ketones and how they are formed.
- What is the significance of 2,4-DNP in testing carbonyl compounds?
- Give one example of an aldol condensation product.
- Mention two industrial uses of aldehydes or ketones.
Fantastic work! You’ve now completed the full journey through aldehydes and ketones — from structure and properties to reactions and applications. You’re building a strong and practical Chemistry foundation that will serve you for years to come. Keep learning with Afrilearn, and you’ll continue to grow into a confident, capable scientist.
