Back to: Organic Chemistry 200 Level
Welcome to class!
Hello bright mind! It’s great to have you here again. You’ve been doing excellently well so far, and today’s lesson is another exciting one. Have you ever smelt kerosene or petrol at a filling station? Or watched a plastic bottle burn and give off black smoke? These everyday experiences are deeply connected to something we’re learning today — Alkenes and Alkynes. Let’s get started and break it down together.
Alkenes And Alkynes
Introduction to Alkenes and Alkynes
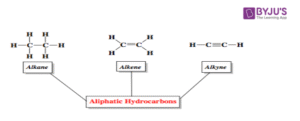
You already know that hydrocarbons are compounds made up of only carbon and hydrogen atoms. In a previous lesson, we looked at alkanes, which are saturated hydrocarbons. Today, we’re talking about their unsaturated cousins — alkenes and alkynes.

The main difference is this: alkenes have at least one double bond between carbon atoms, while alkynes have at least one triple bond. These double and triple bonds give them special chemical behaviours and many practical uses in the real world.
Structure of Alkenes and Alkynes
Alkenes – General formula: CₙH₂ₙ
The simplest alkene is ethene (C₂H₄).
It contains a carbon–carbon double bond (C=C), which makes it more reactive than alkanes.
Alkynes – General formula: CₙH₂ₙ₋₂
The simplest alkyne is ethyne (C₂H₂).
It has a carbon–carbon triple bond (C≡C), which also makes it very reactive.
Think of the double or triple bond like extra “tension” between the carbon atoms, making them more ready to react than the relaxed single bonds in alkanes.
Nomenclature (Naming Alkenes and Alkynes)
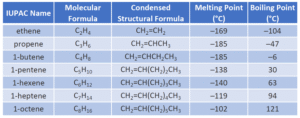
Just like alkanes, we name alkenes and alkynes based on the number of carbon atoms in the longest chain and the position of the double or triple bond.
Examples:
But-1-ene: 4 carbon atoms, double bond starting at position 1.
Pent-2-yne: 5 carbon atoms, triple bond starting at position 2.
Physical Properties
Both alkenes and alkynes are non-polar and insoluble in water but dissolve well in organic solvents.
They are usually gases or low-boiling liquids at room temperature.
As the carbon chain increases, boiling and melting points increase.
Chemical Properties
Because of the multiple bonds, alkenes and alkynes are more reactive than alkanes. They easily take part in addition reactions, where the double or triple bonds open up and allow new atoms to join.
Key Reactions:
Hydrogenation – Adding hydrogen to form an alkane.
Halogenation – Adding halogens like chlorine or bromine.
This is used in testing for unsaturation using bromine water — it turns colourless in the presence of alkenes or alkynes.
Hydration – Adding water in the presence of an acid to form alcohols.
Combustion – Like alkanes, they burn in air but produce a sooty flame due to high carbon content.
Uses of Alkenes and Alkynes
Ethene is used to make polyethene plastics.
Ethyne (acetylene) is used in welding due to its high-temperature flame.
These compounds are also used in making alcohols, detergents, and even drugs.
Summary
- Alkenes contain double bonds and follow the formula CₙH₂ₙ.
- Alkynes contain triple bonds and follow the formula CₙH₂ₙ₋₂.
- Both are unsaturated hydrocarbons and are more reactive than alkanes.
- They take part in addition reactions like hydrogenation, halogenation, and hydration.
- Their uses include fuel, plastics, welding, and manufacturing of various household products.
Evaluation
- What is the difference between alkenes and alkynes?
- Give the general formulas for alkenes and alkynes.
- Describe one test used to detect alkenes or alkynes.
- Mention two uses of alkenes or alkynes in everyday life.
Excellent work! You’ve just mastered another important part of Organic Chemistry — one that shows up in fuels, plastics, and even medicine. Keep going strong, keep learning, and remember that Afrilearn is always here to guide and support your journey. See you in the next exciting lesson!
