Back to: Organic Chemistry 200 Level
Welcome to class!
Hello, great scholar! Today, we’re stepping into the fascinating world of amines — compounds that, though small, are vital in life and industry. From the caffeine in your morning tea to the medicines that keep people healthy, amines are everywhere. Understanding them will open your eyes to the chemistry behind many everyday products.
Amines I
What Are Amines?
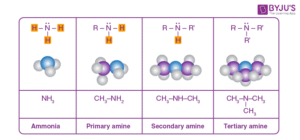
Amines are organic derivatives of ammonia (NH₃), where one or more hydrogen atoms are replaced by alkyl or aryl groups. They contain nitrogen with a lone pair of electrons, making them basic and nucleophilic.
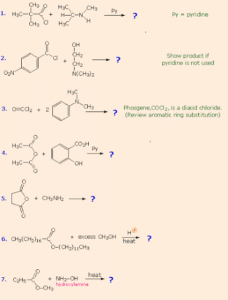
Classification of Amines
Amines are classified based on how many hydrogen atoms are replaced:
Primary Amines (1°) – One hydrogen replaced. Example: CH₃NH₂ (methylamine)
Secondary Amines (2°) – Two hydrogens replaced. Example: (CH₃)₂NH (dimethylamine)
Tertiary Amines (3°) – All three hydrogens replaced. Example: (CH₃)₃N (trimethylamine)
They can also be classified as aliphatic (only alkyl groups) or aromatic (aryl group attached to nitrogen, e.g., aniline).
Physical Properties of Amines
State: Lower aliphatic amines are gases; higher ones are liquids or solids.
Smell: Characteristic fishy odour (less pleasant in higher amines).
Solubility: Lower amines dissolve in water due to hydrogen bonding; solubility decreases with chain length.
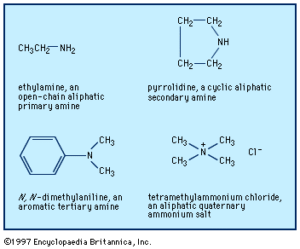
Boiling Points: Higher than alkanes but lower than alcohols of similar size.
Basicity of Amines
The lone pair on nitrogen allows amines to accept protons, making them basic. Their basic strength depends on:
Electron-donating or withdrawing groups.
Solvent effects (amines are more basic in non-aqueous solvents).
Order of basicity in water (generally):
Secondary amines > Primary amines > Tertiary amines > Ammonia.
Preparation of Amines
From Alkyl Halides – Alkyl halide + ammonia → amine (via nucleophilic substitution).
Reduction of Nitriles – R–CN + H₂/Ni → R–CH₂–NH₂.
Reduction of Amides – Using LiAlH₄ to produce amines.
From Nitro Compounds – Reduction with tin and HCl or catalytic hydrogenation.
Summary
- Amines are derivatives of ammonia containing nitrogen with a lone pair.
- Classified into primary, secondary, and tertiary; also aliphatic or aromatic.
- Physical properties are influenced by hydrogen bonding and molecular weight.
- Amines are basic, with secondary amines often being the strongest in aqueous solution.
- They can be prepared from alkyl halides, nitriles, amides, or nitro compounds.
Evaluation
- What is the difference between aliphatic and aromatic amines?
- Arrange primary, secondary, and tertiary amines in order of basicity in water.
- Write the reaction for the preparation of ethylamine from bromoethane.
- State one reason why higher amines are less soluble in water.
Well done! You’ve taken the first step into understanding amines — compounds with big roles in biology, medicine, and materials. With Afrilearn, you’re building knowledge that’s both powerful and practical.
