Back to: Organic Chemistry 200 Level
Welcome to class!
Hello bright mind! Today, we’ll continue our study of amines, but now we’ll focus on their chemical reactions and uses. This is where the theory comes alive — showing you how amines behave in the lab, in nature, and in industry. By the end, you’ll see why chemists value these nitrogen-containing compounds so much.
Amines Il
Chemical Reactions of Amines
Basicity and Salt Formation
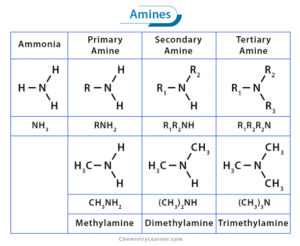
Amines react with acids to form ammonium salts.
Example: CH₃NH₂ + HCl → CH₃NH₃⁺Cl⁻
This property is used to convert volatile amines into non-volatile salts for storage.
Alkylation
Amines react with alkyl halides in nucleophilic substitution, producing higher amines or quaternary ammonium salts.
Example: CH₃NH₂ + CH₃CH₂Br → CH₃NHCH₂CH₃ + HBr
Acylation
Primary and secondary amines react with acid chlorides or anhydrides to form amides.
Example: CH₃NH₂ + CH₃COCl → CH₃NHCOCH₃ + HCl
Reaction with Nitrous Acid (HNO₂)
Primary aliphatic amines: produce alcohols, nitrogen gas, and water.

Example: RNH₂ + HNO₂ → ROH + N₂ + H₂O
Primary aromatic amines: form diazonium salts, important in dye production.
Diazotisation and Coupling Reactions
Aromatic amines like aniline react with nitrous acid at low temperatures to form diazonium salts, which couple with phenols or aromatic amines to form azo dyes.
Oxidation
Amines can be oxidised to various products depending on conditions, such as forming nitro compounds or aldehydes.
Uses of Amines
Pharmaceuticals: Amines are present in many drugs like antihistamines, anaesthetics, and antidepressants.
Dyes: Aromatic amines are used in producing azo dyes for textiles.
Polymers: Nylon production involves amine chemistry.
Agriculture: Many pesticides and herbicides contain amine groups.
Everyday Products: Found in hair dyes, rubber chemicals, and corrosion inhibitors.
Summary
- Amines react with acids, alkyl halides, acid chlorides, and nitrous acid.
- Aromatic amines can undergo diazotisation to make brightly coloured azo dyes.
- Amines are crucial in pharmaceuticals, dyes, polymers, and agriculture.
Evaluation
- Write a balanced equation for the reaction between ethylamine and hydrochloric acid.
- What is the main difference in the reaction of aliphatic and aromatic primary amines with nitrous acid?
- Name two uses of aromatic amines in industry.
- Explain why amines form salts with acids.
Excellent work! You now understand not just what amines are, but how they behave and why they’re so useful. With Afrilearn, you’re connecting classroom chemistry to real-world impact.
