Back to: Organic Chemistry 400 Level
Welcome to class!
Hello intelligent learner, I’m really happy to see you back today. I hope you’re feeling motivated and ready, because our new lesson — Amino Acids and Proteins I — is a very important and foundational topic in organic and biological chemistry. You will soon see how the food we eat is converted into important biomolecules that build our muscles, transport oxygen and even control chemical reactions inside the body.
Amino Acids and Proteins I
Have you ever wondered why athletes take protein shakes after training, or why beans are considered a “body-building” food in most Nigerian homes? It’s because of the presence of amino acids and proteins — the essential building blocks of life.
Meaning of Amino Acids
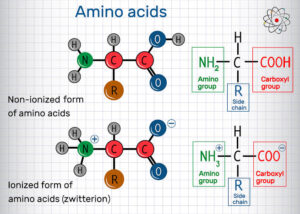
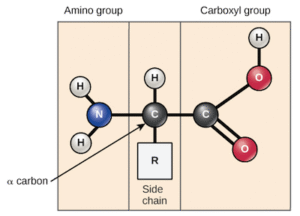
Amino acids are organic compounds that contain two functional groups in the same molecule:
an amino group (–NH₂) and
a carboxyl group (–COOH).
They are the basic units that combine together to form proteins. There are 20 standard amino acids found in proteins, and each has a different side chain (R-group) which determines its physical and chemical properties.
General Structure of Amino Acids
The general structure is:
H₂N – CH(R) – COOH
Where R can be a simple hydrogen (as in glycine) or a more complex side chain (as in phenylalanine).
Most amino acids (except glycine) are chiral, meaning they exist as enantiomers and can rotate plane-polarised light.
Classification of Amino Acids
Amino acids can be classified based on the nature of the R-group:
Non-polar (hydrophobic) – e.g., alanine, valine, leucine
Polar (hydrophilic) – e.g., serine, threonine
Acidic – contain an extra carboxyl group (e.g., aspartic acid, glutamic acid)
Basic – contain an extra amino group (e.g., lysine, arginine)
Aromatic – contain an aromatic ring (e.g., phenylalanine, tyrosine, tryptophan)
You can compare this to a classroom where students have different personalities; some are calm (non-polar), some are outspoken (basic), some are gentle (polar) and some are dramatic (aromatic)!
Essential vs Non-Essential Amino Acids
Essential amino acids cannot be synthesised by the human body and must be obtained from food (e.g., lysine, methionine, tryptophan).
Non-essential amino acids can be produced by the body (e.g., alanine, glycine).
This is why a balanced diet that includes foods like eggs, fish, beans and meat is so important.
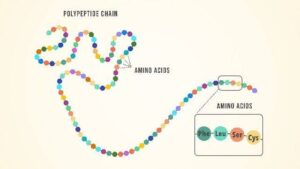
Peptide Bond Formation
Amino acids link together through peptide bonds, which form between the carboxyl group of one amino acid and the amino group of another with the elimination of a water molecule (condensation reaction).
Two amino acids form a dipeptide, three form a tripeptide, and many together form a polypeptide, which eventually folds to give a functional protein.
Think of it like a necklace: each bead is an amino acid and the string connecting them is the peptide bond. When you have a long chain of beads, it becomes a beautiful necklace – just like proteins are long chains of amino acids.
Functions of Amino Acids
Building blocks of proteins
Sources of energy when the body runs out of carbohydrates
Precursors for hormones, neurotransmitters and other biomolecules
Help in wound healing and immune function
Summary
- Amino acids are organic molecules containing both an amino group and a carboxyl group.
- There are 20 standard amino acids, classified according to the properties of their side chains.
- Essential amino acids must be obtained from food, while non-essential ones are synthesised in the body.
- Amino acids join through peptide bonds to form polypeptides and proteins.
- Amino acids play important roles in energy production, protein synthesis and several metabolic processes.
Evaluation
- Define amino acids and state the two functional groups they contain.
- Differentiate between essential and non-essential amino acids with one example each.
- What is a peptide bond and how is it formed?
- List two biological functions of amino acids.
Fantastic effort today! You’re building a strong foundation in biochemical chemistry and Afrilearn is proud of your dedication. Keep going — your next lesson will take you deeper into the fascinating world of proteins!
