Back to: Organic Chemistry 200 Level
Welcome to class!
Hi there, brilliant learner! I’m so glad to see you here again. You’ve already learnt a lot about different types of organic compounds, but today’s lesson introduces you to a special class of compounds that smell sweet, behave differently, and show up in everything from perfumes to petrol. These are called Aromatic Compounds — and no, it’s not just because of their smell! Let’s break it down together in the simplest way.
Aromatic Compounds I
What Are Aromatic Compounds?
The word “aromatic” may remind you of something that smells nice — like perfume, flowers, or even pepper soup! While some aromatic compounds do have strong smells, in Chemistry, the name refers to compounds that contain a special kind of stable ring structure — not just their scent.
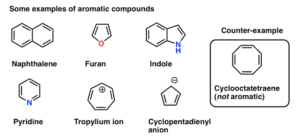
Aromatic compounds are cyclic (ring-shaped), planar (flat), and contain delocalised electrons shared across the ring. This gives them unusual stability, which we call aromaticity.
The most common and simplest aromatic compound is benzene.
Structure of Benzene
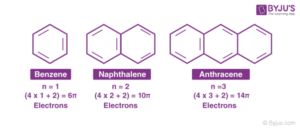
Let’s talk about benzene (C₆H₆), the parent compound of the aromatic family.
Benzene has six carbon atoms joined in a ring.
Each carbon forms three bonds — two with neighbouring carbon atoms and one with a hydrogen atom.
The fourth electron from each carbon is shared (delocalised) across the ring.
This creates a cloud of electrons above and below the ring, making the structure extra stable.
Scientists used to draw benzene with alternating single and double bonds, but we now represent it with a hexagon and a circle inside to show the delocalised electrons.
Aromaticity – Why Is Benzene So Stable?
Benzene doesn’t behave like other alkenes, even though it has what looks like double bonds. For example:
It does not decolourise bromine water, unlike normal alkenes.
It doesn’t take part in addition reactions easily.
Instead, it prefers substitution reactions to keep its stable ring intact.
This special stability is what we call aromaticity — it comes from the delocalised electrons being evenly shared in the ring.
Examples of Aromatic Compounds
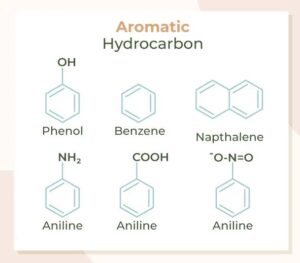
Besides benzene, here are a few other aromatic compounds:
Toluene (methylbenzene) – Found in paint thinners.
Phenol (hydroxybenzene) – Used in antiseptics.
Aniline (aminobenzene) – Used in making dyes.
Naphthalene – Found in mothballs and air fresheners.
All these compounds have benzene rings as part of their structure.
Sources of Aromatic Compounds
Aromatic compounds can be found in both natural and man-made sources:
Natural: Found in plant oils, coal tar, and petroleum.
Synthetic: Produced in industries for making plastics, dyes, medicines, and detergents.
Importance of Aromatic Compounds
Aromatic compounds are very useful in everyday life:
They’re used in making plastics, dyes, drugs, perfumes, detergents, and explosives.
They are also found in fuels like petrol, contributing to energy production.
Summary
- Aromatic compounds are cyclic, stable, and contain delocalised electrons.
- Benzene is the simplest aromatic compound with six carbon atoms in a ring.
- Aromaticity gives benzene unique stability and prevents it from reacting like normal alkenes.
- Examples include toluene, phenol, and naphthalene.
- Aromatic compounds are found in nature and are widely used in industry.
Evaluation
- What is the meaning of aromaticity in Chemistry?
- Describe the structure of benzene and explain why it is stable.
- Why doesn’t benzene react like a typical alkene?
- Mention three uses of aromatic compounds in everyday life.
Fantastic job! You’ve just been introduced to one of the most fascinating families in Organic Chemistry — the aromatics. They are not only important in science but also deeply woven into our daily lives. Keep this curiosity alive and remember, with Afrilearn, you have all the support you need to understand, succeed, and shine. See you in the next lesson!
