Back to: Organic Chemistry 200 Level
Welcome to class!
Hello brilliant scholar! It’s great to see you here again, ready to learn. Today, we’re moving into another important family of organic compounds — Carboxylic Acids. You’ve definitely encountered them before, even if you didn’t know their names. The sour taste of vinegar, the sharp smell of spoiled milk, and the tangy flavour of citrus fruits — all these are due to carboxylic acids. Let’s get to know them better.
Carboxylic Acids I
Structure and Functional Group
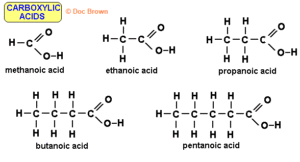
Carboxylic acids contain the carboxyl group (-COOH), which is made up of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom. This unique arrangement is what gives them their acidic properties.
General Formula
For a straight-chain carboxylic acid: CₙH₂ₙO₂
Example: Methanoic acid (HCOOH), Ethanoic acid (CH₃COOH)
Naming Carboxylic Acids (IUPAC Rules)
Identify the longest continuous carbon chain containing the -COOH group.
Replace the -e ending of the corresponding alkane with -oic acid.
Example: Methane → Methanoic acid, Propane → Propanoic acid.
Numbering starts from the carbon in the carboxyl group (this carbon is always C-1).
Physical Properties
Boiling Points: Carboxylic acids have higher boiling points than similar-sized alcohols due to strong hydrogen bonding between molecules.
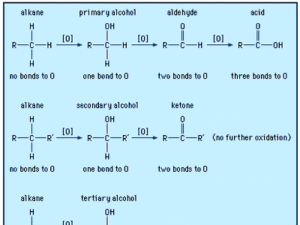
Solubility: Lower members are highly soluble in water because they form hydrogen bonds with water. Solubility decreases as the carbon chain gets longer.
Odour: Many have strong, sharp odours — ethanoic acid smells like vinegar.
Acidic Nature
Carboxylic acids are weak acids. In water, they partially ionise:
CH₃COOH ⇌ CH₃COO⁻ + H⁺
Their acidity is due to the ability of the carboxyl group to stabilise the negative charge on the conjugate base (carboxylate ion) through resonance.
Occurrence in Everyday Life
Methanoic acid: Found in ant and bee stings.
Ethanoic acid: Main component of vinegar, used in cooking and food preservation.
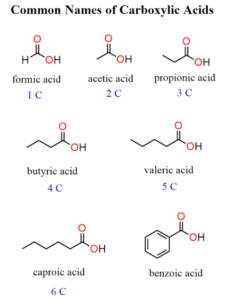
Citric acid: Found in citrus fruits like oranges, lemons, and limes.
Summary
- Carboxylic acids contain the carboxyl (-COOH) functional group.
- Named by replacing the alkane ending with -oic acid.
- High boiling points and solubility due to hydrogen bonding.
- They are weak acids, partially ionising in water.
- Common in nature and everyday products.
Evaluation
- Draw the structure of propanoic acid.
- Why do carboxylic acids have higher boiling points than alcohols?
- Write the equation for the ionisation of ethanoic acid in water.
- Give two examples of natural sources of carboxylic acids.
Well done! You’ve just taken the first step in understanding carboxylic acids — an important family of compounds with a presence in your kitchen, nature, and industry. Keep going with Afrilearn, and you’ll soon see how their reactions make them even more fascinating.
