Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my ever-ready learner! I’m glad to have you here again, eager to add another building block to your organic chemistry knowledge. Today, we begin our journey into carboxylic acid derivatives — an exciting group of compounds that play important roles in medicine, food, industry, and nature. By the end of this lesson, you’ll see why understanding them is like unlocking another set of keys in organic chemistry.
Carboxylic Derivatives I
Meaning of Carboxylic Acid Derivatives
Carboxylic acid derivatives are compounds that can be thought of as carboxylic acids where the –OH group is replaced by another group such as –Cl, –OR, –OCOR, or –NH₂. These include acyl chlorides, esters, acid anhydrides, and amides. They all share the same carbonyl group (C=O) directly attached to a group derived from the acid.
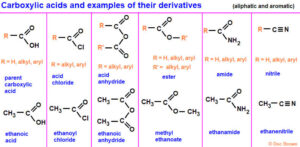
Types of Carboxylic Acid Derivatives
Acid chlorides (R–COCl): Highly reactive, used in synthesis of esters and amides.
Esters (R–COOR’): Found in natural flavours and fragrances; often responsible for fruity smells.
Acid anhydrides (R–CO–O–CO–R’): Used in acetylation reactions and as intermediates in industrial synthesis.
Amides (R–CONH₂, R–CONHR’, R–CONR₂): Common in proteins, medicines, and synthetic materials like nylon.
General Methods of Preparation
Carboxylic acid derivatives can be prepared from carboxylic acids using specific reagents:
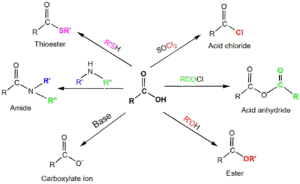
Acid chlorides from carboxylic acids using thionyl chloride (SOCl₂).
Esters from acids and alcohols via esterification with an acid catalyst.
Acid anhydrides from dehydration of carboxylic acids.
Amides from reaction of acids (or acid chlorides) with ammonia or amines.
Relative Reactivity
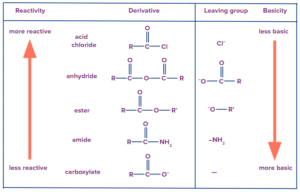
The reactivity of carboxylic acid derivatives depends on the nature of the group attached to the carbonyl. In general, the order of reactivity is:
Acid chlorides > Acid anhydrides > Esters > Amides.
This reactivity pattern is due to differences in electron-withdrawing ability and leaving group stability.
Physical Properties
Most carboxylic derivatives are polar and have boiling points influenced by hydrogen bonding and molecular weight. Amides often have higher melting and boiling points due to strong hydrogen bonding, while acid chlorides are typically liquids with pungent odours.
Uses in Nigerian and Global Contexts
Esters are widely used in making perfumes, soaps, and food flavourings in Nigeria’s cosmetic and food industries.
Acid chlorides are used in pharmaceutical synthesis for producing drugs like aspirin.
Amides form the backbone of proteins and synthetic fibres, important in textile production.
Ethanoic acid reacts with SOCl₂ to give ethanoyl chloride, which can then react with ethanol to produce ethyl ethanoate — a fruity-smelling ester used in flavouring drinks.
Summary
- Carboxylic acid derivatives share a carbonyl group attached to a substituent replacing the –OH of carboxylic acids.
- Main types include acid chlorides, esters, acid anhydrides, and amides.
- They can be prepared from carboxylic acids using specific reagents like SOCl₂, alcohols, or amines.
- Reactivity order: Acid chlorides > Acid anhydrides > Esters > Amides.
- They have wide uses in food, pharmaceuticals, cosmetics, and industry.
Evaluation
- Define a carboxylic acid derivative and give two examples.
- What reagent is commonly used to prepare acid chlorides from carboxylic acids?
- Arrange these derivatives in order of decreasing reactivity: amides, esters, acid chlorides, acid anhydrides.
- Mention one use of esters in the Nigerian food industry.
- Explain why amides have higher melting points than esters.
You are building a strong foundation in organic chemistry, learning how one functional group can lead to many important and useful compounds. Afrilearn is proud to see you gaining knowledge that connects directly to everyday life and industry.
