Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my focused scholar! It’s great to have you back for the second part of our journey into carboxylic acid derivatives. In the first lesson, we met the family members of this group; now, we will get to know how they react, the factors influencing those reactions, and their practical uses in the real world. By the end of today’s lesson, you will not just recognise these compounds — you will understand how to make them work for you in synthesis.
Carboxylic Derivatives II
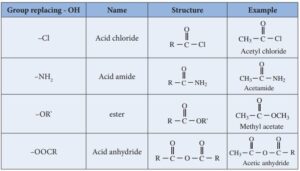
Nucleophilic Acyl Substitution – The Core Reaction
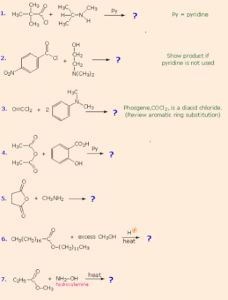
The most important reaction of carboxylic acid derivatives is nucleophilic acyl substitution. In this reaction, a nucleophile attacks the carbonyl carbon, the double bond to oxygen temporarily breaks, and a leaving group departs, restoring the carbonyl. The nature of the leaving group determines the ease of the reaction.
Reaction Mechanism (General Steps)
Step 1: Nucleophile attacks the electrophilic carbonyl carbon.
Step 2: Tetrahedral intermediate forms.
Step 3: Leaving group departs, regenerating the carbonyl.
This is the reason acid chlorides, with –Cl as a strong leaving group, are so reactive.
Hydrolysis of Carboxylic Acid Derivatives
Acid chlorides hydrolyse rapidly in water to give carboxylic acids.
Esters undergo acid or base hydrolysis to give acids and alcohols (in base, this is called saponification).
Amides require stronger heating with acid or base for hydrolysis.
In Nigerian soap-making industries, ester saponification is the key step in producing soap from vegetable oils.
Reduction of Carboxylic Acid Derivatives
LiAlH₄ reduces esters and acid chlorides to primary alcohols.
NaBH₄ is milder and generally ineffective for esters and amides.
Amides can be reduced to amines by LiAlH₄.
For instance, in pharmaceutical synthesis, amide reduction can yield amine-based drugs.
Reactions with Ammonia and Amines
Acid chlorides and anhydrides react readily with ammonia to produce primary amides, and with primary/secondary amines to produce N-substituted amides. This is used in producing certain agrochemicals and textile fibres.
Transesterification
Esters can be converted to other esters by reaction with a different alcohol, usually in the presence of an acid or base catalyst. This method is important in biodiesel production, where vegetable oil esters are converted to methyl esters for use as fuel.
Industrial Applications
Acid chlorides are used in making acylated dyes and pharmaceuticals.
Esters are vital for perfumes, food flavourings, and solvents.
Amides are the backbone of synthetic fibres like nylon, important in Nigeria’s textile industry.
Anhydrides like acetic anhydride are used in making aspirin and cellulose acetate.
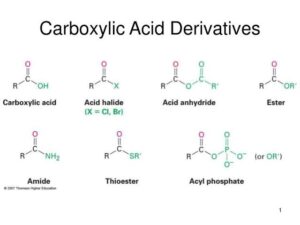
When benzoyl chloride reacts with ammonia, benzamide is formed along with the release of hydrogen chloride gas. This reaction demonstrates the nucleophilic acyl substitution pathway with ammonia as the nucleophile.
Summary
- Carboxylic acid derivatives primarily undergo nucleophilic acyl substitution reactions.
- The leaving group’s strength determines the compound’s reactivity.
- Hydrolysis can occur under acidic or basic conditions, with varying ease depending on the derivative.
- LiAlH₄ is a powerful reducing agent for esters, acid chlorides, and amides.
- Industrial applications range from soap-making to drug manufacturing and textile production.
Evaluation
- What is the main reaction mechanism of carboxylic acid derivatives?
- Arrange these derivatives in order of decreasing reactivity towards nucleophilic acyl substitution: esters, amides, acid chlorides.
- Which reducing agent can convert an amide into an amine?
- What is the name of the base-catalysed hydrolysis of esters?
- Give one industrial use of acid anhydrides.
Your understanding of carboxylic acid derivatives is becoming richer and more practical. With Afrilearn, you are mastering reactions that form the basis of many industrial and biological processes, preparing you for excellence in both academic and professional chemistry.
