Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my ever-curious learner! Today, we are stepping into an area of organic chemistry that focuses on the shapes molecules adopt and how these shapes affect their behaviour. This is called Conformational Analysis. You’ll soon see that even when the atoms in a molecule are connected in the same way, the positions they take in space can change, almost like people sitting in different positions on a bus.
Conformational Analysis I
Meaning of Conformational Analysis
Conformational analysis is the study of the different spatial arrangements that a molecule can adopt due to rotation around single (sigma) bonds, and how these arrangements influence the molecule’s stability and properties. These different shapes are called conformations, and unlike stereoisomers, they can interconvert freely at room temperature.
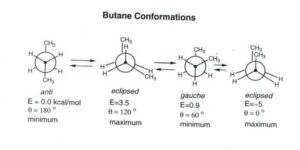
Conformations in Ethane
Ethane (C₂H₆) is the simplest example to understand conformations. When you rotate one carbon atom relative to the other around the C–C bond, the hydrogen atoms change positions in space. Two key conformations occur repeatedly: the staggered conformation, where hydrogen atoms on one carbon are positioned as far apart as possible from the hydrogens on the other carbon; and the eclipsed conformation, where hydrogens line up directly behind one another when viewed along the C–C bond.
Staggered vs Eclipsed Conformations
The staggered conformation is more stable because it minimises electron cloud repulsion between the bonds. The eclipsed conformation is less stable because the bonds are closer together, increasing repulsion. The energy difference between these conformations is called torsional strain. Molecules naturally prefer the conformation with the lowest energy.
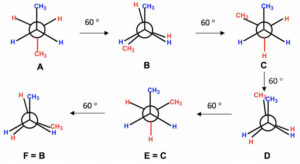
Newman Projections
To clearly visualise conformations, chemists often use Newman projections. In this view, you look straight down the bond between two atoms, with the front atom represented as a dot and the back atom as a circle. This makes it easier to compare the positions of substituents and analyse strain.
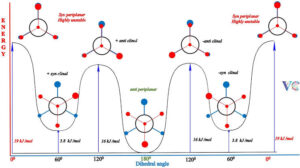
Importance of Conformational Analysis
Understanding conformations helps explain why certain reactions happen faster or slower, why some molecules are more stable than others, and how large molecules like proteins fold into specific shapes. This knowledge is key in designing drugs, materials, and even predicting the taste and smell of compounds.
Summary
- Conformational analysis studies different spatial arrangements of atoms due to rotation around single bonds.
- Conformations interconvert freely at room temperature, unlike stereoisomers.
- In ethane, staggered conformations are more stable than eclipsed due to reduced electron repulsion.
- Torsional strain is the extra energy in eclipsed conformations caused by bond repulsion.
- Newman projections help visualise conformations and analyse stability.
Evaluation
- Define conformational analysis.
- What is the main difference between conformations and stereoisomers?
- Which is more stable in ethane: staggered or eclipsed conformation, and why?
- What is torsional strain?
- What is the purpose of a Newman projection?
You are becoming a true organic chemistry thinker — seeing molecules not just as flat formulas, but as dynamic 3D structures in motion. Afrilearn celebrates your progress, and you are building skills that will make you stand out in your studies and your future scientific career.
