Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my brilliant learner! I’m excited to see you back for the second part of Conformational Analysis. Last time, we learnt that molecules can adopt different shapes (conformations) due to rotation around single bonds, and that some conformations are more stable than others. Today, we will look at more complex molecules, learn how to use energy diagrams to compare their stability, and understand why bulky groups play a big role in determining the preferred shape of a molecule.
Conformational Analysis II
Conformations in Propane and Butane
In propane (C₃H₈), the situation is similar to ethane, but the presence of a methyl group changes the pattern slightly. The staggered conformation is still more stable than the eclipsed one because it minimises repulsion, but now the eclipsed form is less stable when a methyl group is directly behind a hydrogen compared to hydrogen-hydrogen eclipsing — methyl groups have more electrons and take up more space, causing extra strain.

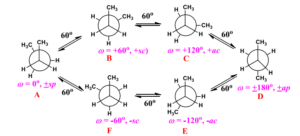
In butane (C₄H₁₀), rotation around the central C–C bond gives rise to several key conformations: the anti conformation, where the two methyl groups are 180° apart (most stable); the gauche conformation, where methyl groups are 60° apart (slightly less stable due to steric hindrance); and the eclipsed conformations, where groups are directly aligned (least stable).
Steric Hindrance
Steric hindrance occurs when bulky groups are too close to each other, leading to repulsion between their electron clouds. This is one of the key factors in conformational stability — the larger the groups, the more they “push” each other apart. This explains why the anti conformation of butane is more stable than the gauche form.
Cyclohexane Conformations
Cyclohexane (C₆H₁₂) is a ring system that avoids angle strain by adopting non-planar shapes. The most important conformations are the chair form, boat form, and twist-boat form. The chair conformation is the most stable because it minimises both angle strain and torsional strain. In the chair form, there are two types of positions for substituents: axial (pointing up or down along the ring axis) and equatorial (pointing outward around the ring). Large groups prefer the equatorial position because it reduces steric hindrance.
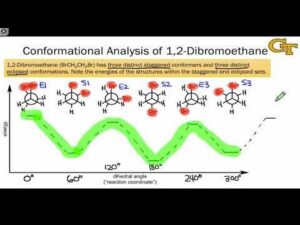
Energy Diagrams
An energy diagram for conformations shows how stability changes as the molecule rotates. The most stable conformations correspond to the lowest points (energy minima), while the least stable ones are at the highest points (energy maxima). This allows chemists to predict which shapes will dominate at room temperature and which will only appear under special conditions.
Importance in Real Life
Conformational preferences affect how enzymes recognise molecules, how drugs fit into their targets, and how materials bend or stretch. In perfume chemistry, slight differences in conformation can change how a scent is perceived. In agriculture, the effectiveness of pesticides can depend on the molecule’s preferred shape.
Summary
- Propane’s conformations are similar to ethane’s, but methyl-hydrogen eclipsing causes more strain than hydrogen-hydrogen eclipsing.
- Butane has anti, gauche, and eclipsed conformations; anti is the most stable due to minimal steric hindrance.
- Steric hindrance is repulsion between bulky groups that are too close.
- Cyclohexane adopts chair, boat, and twist-boat conformations, with chair being the most stable.
- Energy diagrams show stability patterns across different conformations.
Evaluation
- Which is the most stable conformation of butane and why?
- What is steric hindrance?
- Name the three main conformations of cyclohexane.
- Why do bulky groups prefer the equatorial position in cyclohexane?
- What information does an energy diagram of conformations provide?
You are doing amazingly well in building a clear mental picture of molecules in motion. This ability to “see” chemistry in 3D will make you a sharper thinker and a better scientist. Afrilearn is proud of your progress, and this knowledge will serve you well in every area of organic chemistry.
