Back to: MICROBIOLOGY 200 LEVEL
Welcome to class!
Hello there, superstar! I’m really glad you’re here again—ready to grow, learn, and master the wonders of life through microbiology. Today’s lesson is all about Deamination and Transamination in Protein Metabolism. Yes, the names might sound a bit serious, but they are simply ways your body handles proteins, especially when it needs to use them for energy. Let’s walk through it together in a way that feels just like home.
Deamination And Transamination In Protein Metabolism
What is Protein Metabolism?
You already know that proteins are one of the most important nutrients we get from foods like beans, meat, eggs, and fish. Normally, proteins help build and repair tissues. But sometimes, when the body needs energy or when there’s extra protein, the body breaks them down. The way this happens is through deamination and transamination.
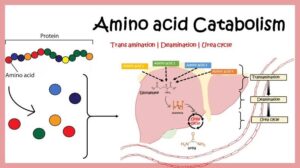
Let’s understand them one at a time.
Transamination
Transamination is like a friendly exchange between amino acids and another molecule.
Each amino acid has an amino group (–NH₂).
In transamination, this amino group is transferred from one amino acid to a keto acid (a molecule without an amino group), forming a new amino acid and a new keto acid.
It’s like in a Nigerian classroom where students exchange textbooks—one student gives a book (amino group) to another who didn’t have one (keto acid), and now both have something useful.
This process helps the body:
Produce non-essential amino acids (those the body can make itself).
Recycle amino groups.
Prepare amino acids for further breakdown if energy is needed.
Enzymes involved: Aminotransferases (also called transaminases). These are like the teachers guiding the textbook exchange.
Deamination
Now, deamination is when the amino group is completely removed from an amino acid.
The removed amino group becomes ammonia (NH₃), which is toxic if it builds up.
The body quickly converts ammonia into urea (in the liver), which is then removed from the body in urine.
This is how the body safely disposes of extra nitrogen from proteins.
The remaining part of the amino acid (the carbon skeleton) can now be:
Converted to glucose (for energy)
Used in the Krebs cycle
Stored as fat
Where Do These Happen?
Transamination: Mostly happens in the liver and muscle cells.
Deamination: Mainly takes place in the liver.
Think of transamination as a friend borrowing your generator to power their house. You both benefit. But deamination is like removing the engine from the generator completely—useful if you want to scrap it or reuse the parts differently, but you can’t power anything with it anymore.
Why Are These Processes Important?
They allow the body to use proteins for energy when needed.
Help in removing excess nitrogen safely.
Play a big role in the metabolism of amino acids.
Support the body in maintaining a proper nitrogen balance.
Summary
- Transamination is the transfer of an amino group to form new amino acids.
- Deamination is the removal of an amino group, leading to the formation of ammonia and urea.
- Both processes occur mainly in the liver and help manage protein metabolism.
- They help in energy production, nitrogen balance, and amino acid recycling.
Evaluation
- What is the difference between deamination and transamination?
- Why is ammonia dangerous to the body?
- What happens to the amino acid after deamination?
- Where does transamination commonly take place?
You are doing wonderfully well! Protein metabolism might sound complex, but look at how smoothly you’ve understood it. Keep up this energy, because learning with Afrilearn is all about building confidence, curiosity, and the future you deserve. Let’s keep moving forward together—you’re doing excellently!
