Back to: Organic Chemistry 200 Level
Welcome to class!
Hello, bright mind! It’s always a joy to see you eager to learn. Today, we’re going to look at derivatives of carboxylic acids — special compounds that come from carboxylic acids when the –OH group is replaced with another atom or group. Think of them like “relatives” of carboxylic acids that have their own personalities and unique uses in everyday life, from medicines to fabrics.
Derivatives Of Carboxylic Acids I
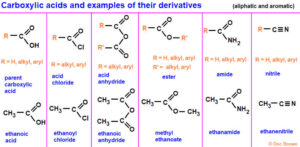
What Are Carboxylic Acid Derivatives?
Carboxylic acid derivatives are compounds that can be converted back to carboxylic acids through hydrolysis. They contain the acyl group (R–CO–) and differ based on what replaces the –OH in the acid group.
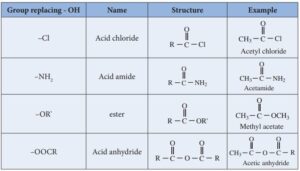
The main derivatives are:
Acid Chlorides (R–COCl)
Acid Anhydrides (R–CO–O–CO–R)
Esters (R–COOR’)
Amides (R–CONH₂, R–CONHR, or R–CONR₂)
Formation of Acid Chlorides
Produced by reacting carboxylic acids with chlorinating agents such as phosphorus pentachloride (PCl₅), phosphorus trichloride (PCl₃), or thionyl chloride (SOCl₂):
CH₃COOH + PCl₅ → CH₃COCl + POCl₃ + HCl
Acid chlorides are very reactive and are key starting materials for making esters and amides.
Formation of Acid Anhydrides
Can be made by heating two carboxylic acid molecules together:
2CH₃COOH → (CH₃CO)₂O + H₂O
Industrially, they can also be prepared by reacting acid chlorides with sodium salts of carboxylic acids.
Commonly used in the manufacture of aspirin.
Formation of Esters
Prepared by reacting a carboxylic acid with an alcohol in the presence of concentrated sulphuric acid (esterification):
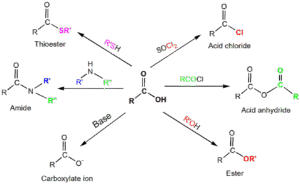
CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O
Esters are responsible for many pleasant fruity smells and flavours.
Formation of Amides
Produced by reacting acid chlorides or anhydrides with ammonia or amines:
CH₃COCl + NH₃ → CH₃CONH₂ + HCl
Naturally present in proteins as part of peptide bonds.
Summary
- Carboxylic acid derivatives share the acyl group and can be hydrolysed back to carboxylic acids.
- Main types: acid chlorides, anhydrides, esters, and amides.
- They are formed by replacing the –OH group of the acid with another atom or group.
- Widely used in medicine, food flavouring, perfumes, and synthetic materials.
Evaluation
- Name four derivatives of carboxylic acids and give one example of each.
- Write the balanced equation for the formation of ethanoyl chloride from ethanoic acid.
- State one industrial use of acid anhydrides.
- What group replaces –OH in an amide?
Excellent work today! You’ve taken the first step in understanding a family of compounds that plays a huge role in chemistry and industry. With Afrilearn by your side, every topic becomes clearer, and your confidence grows with each lesson.
