Back to: Organic Chemistry 200 Level
Welcome to class!
Hello, brilliant student! Today, we continue our journey into the world of carboxylic acid derivatives, but this time, we’ll focus on their reactions and reactivity trends. Understanding these reactions is key to mastering organic synthesis and seeing how chemists create products that touch our daily lives — from medicines to fragrances.
Derivatives Of Carboxylic Acids II
Reactivity of Carboxylic Acid Derivatives
Carboxylic acid derivatives undergo nucleophilic acyl substitution, where a nucleophile attacks the carbonyl carbon, replacing the existing group. The ease of this reaction depends on the leaving group and the stability of the derivative.
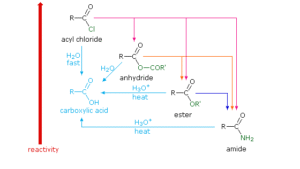
The general reactivity order is:
Acid Chlorides > Acid Anhydrides > Esters ≈ Carboxylic Acids > Amides
Acid chlorides: Most reactive due to the strong electron-withdrawing effect of chlorine and poor resonance stabilisation.
Amides: Least reactive because the nitrogen donates electrons into the carbonyl, making it less electrophilic.
Important Reactions of Carboxylic Acid Derivatives
Hydrolysis
Converts derivatives back to carboxylic acids.
For acid chlorides: CH₃COCl + H₂O → CH₃COOH + HCl
For amides: Requires heat and acid or base.
Reaction with Alcohols (Esterification)
Acid chlorides or anhydrides react with alcohols to form esters.
CH₃COCl + C₂H₅OH → CH₃COOC₂H₅ + HCl
Reaction with Ammonia and Amines (Amidation)
Forms amides when reacted with ammonia or amines.
CH₃COCl + NH₃ → CH₃CONH₂ + HCl
Transesterification
Conversion of one ester to another by reacting with an alcohol.
CH₃COOC₂H₅ + CH₃OH → CH₃COOCH₃ + C₂H₅OH
Reduction
LiAlH₄ can reduce esters and amides to alcohols or amines respectively.
Industrial and Everyday Applications
Acid chlorides: Used in pharmaceutical synthesis and making acylated products like perfumes.
Acid anhydrides: Key in producing aspirin.
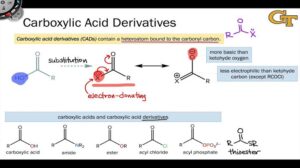
Esters: Give fruity flavours and fragrances used in foods and cosmetics.
Amides: Found in nylon, proteins, and some medicines.
Summary
- All carboxylic acid derivatives undergo nucleophilic acyl substitution.
- Their reactivity depends on the nature of the leaving group, with acid chlorides being the most reactive and amides the least.
- They participate in important reactions like hydrolysis, esterification, amidation, transesterification, and reduction.
Evaluation
- Arrange acid chlorides, amides, esters, and anhydrides in order of reactivity.
- What type of reaction is common to all carboxylic acid derivatives?
- Write the reaction between ethanoyl chloride and ammonia.
- State one industrial use of esters.
You’ve just mastered the key reactions of carboxylic acid derivatives — a foundation for advanced organic synthesis. Keep up the momentum, because with Afrilearn, every topic is a step closer to mastering your craft.
