Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always wonderful to learn with you. Imagine standing outside during harmattan season in Nigeria. If the cold wind is blowing, you naturally draw closer to your friend for warmth. In a similar way, molecules that have positive and negative “ends” are attracted to each other. This special type of attraction is what we call dipole–dipole interactions.
Dipole-dipole Interactions
What are Dipole–Dipole Interactions?
Dipole–dipole interactions are forces of attraction that occur between molecules that have permanent dipoles—that is, molecules with a partial positive charge on one side and a partial negative charge on the other.
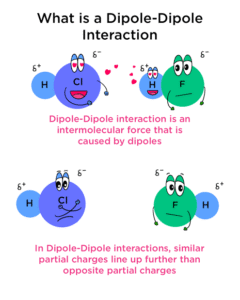
A dipole happens when electrons are not shared equally in a covalent bond. For example, in hydrogen chloride (HCl), chlorine is more electronegative, so it pulls the electrons closer to itself. This makes chlorine slightly negative (δ–) and hydrogen slightly positive (δ+). When two HCl molecules come close, the δ+ hydrogen of one molecule is attracted to the δ– chlorine of another.
Key Features of Dipole–Dipole Interactions
They only occur in polar molecules (molecules with permanent dipoles).
The strength of the interaction depends on the polarity of the molecules—the greater the electronegativity difference, the stronger the attraction.
These forces are stronger than van der Waals (London dispersion forces), but weaker than hydrogen bonds.
Examples in Everyday Life
Hydrogen Chloride (HCl): Molecules are held together by dipole–dipole interactions, giving HCl a higher boiling point compared to non-polar gases like Cl₂.
Acetone (CH₃COCH₃): Acetone, often used as nail polish remover, has dipole–dipole forces between its molecules, making it more volatile than water but less so than non-polar solvents like petrol.
Cooking Oil vs. Water: Water molecules have strong dipole–dipole interactions (plus hydrogen bonding), which is why they don’t mix with non-polar oils that lack dipoles.
Why Are Dipole–Dipole Interactions Important?
They affect boiling points: Polar molecules with dipole–dipole interactions have higher boiling points than non-polar molecules of similar size.
They affect solubility: Polar substances dissolve better in polar solvents (like sugar in water), while non-polar ones dissolve better in non-polar solvents (like oil in petrol).

They influence the physical state of substances: Molecules with stronger dipole–dipole interactions are often liquids or solids at room temperature.
Summary
- Dipole–dipole interactions occur between polar molecules with permanent positive and negative ends.
- They are stronger than dispersion forces but weaker than hydrogen bonds.
- They explain why polar molecules often have higher boiling points and different solubilities compared to non-polar molecules.
Evaluation
- What is a dipole–dipole interaction in simple terms?
- Give two examples of substances where dipole–dipole forces are important.
- Compare the strength of dipole–dipole interactions with hydrogen bonding.
- Why does HCl have a higher boiling point than Cl₂?
Fantastic work today! Remember, every concept you learn brings you closer to mastering Chemistry and the world around you. Keep shining—Afrilearn is proud of your progress.
