Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m really glad to have you here today. Let me start with something you can picture clearly. Imagine you are in a crowded concert in Lagos, and your best friend is calling you from the front stage. Even though you want to hear them, the noise and chatter of the people in between make it harder to catch their voice. In the atom, electrons feel the same way—the nucleus is “calling” them with its positive charge, but other electrons in between block or weaken this pull. This brings us to two important ideas: effective nuclear charge and shielding.
Effective Nuclear Charge And Shielding
What is Nuclear Charge?
Nuclear charge is simply the total positive charge of the nucleus, which equals the number of protons. For example, sodium has 11 protons, so its nuclear charge is +11.
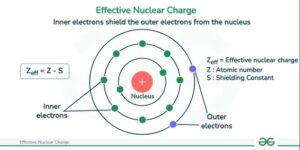
Effective Nuclear Charge (Zeff)
However, electrons don’t always feel the full pull of the nucleus. The effective nuclear charge is the net positive charge experienced by an electron in an atom after accounting for the repelling effects of other electrons.
Think of it like this: your parents may want to give you direct instructions, but if your siblings keep interrupting, you don’t feel the full message. The interruptions reduce the “effective” message you receive.
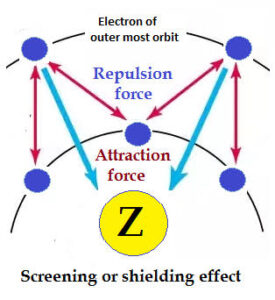
Shielding (or Screening Effect)
Shielding is the blocking of the attractive force of the nucleus on outer electrons by inner electrons.
Inner electrons act like a shield, reducing the nuclear pull felt by the outermost (valence) electrons.
For example, in sodium (1s² 2s² 2p⁶ 3s¹), the single 3s electron does not feel the full +11 from the nucleus. The 10 inner electrons in the 1s, 2s, and 2p orbitals shield it, so the effective nuclear charge is much less than +11.
Trends in the Periodic Table
Across a Period (left to right): Effective nuclear charge increases because more protons are added, but shielding remains almost the same. This is why atoms become smaller across a period.
Down a Group: Effective nuclear charge increases only slightly, but shielding increases greatly because more electron shells are added. This is why atoms become larger down a group.
Why Is This Important?
Effective nuclear charge and shielding explain many chemical properties, including:
Atomic size (why atoms shrink across a period but expand down a group).
Ionisation energy (why it’s harder or easier to remove electrons).
Reactivity trends in metals and non-metals.
Summary
- Nuclear charge = number of protons in the nucleus.
- Effective nuclear charge (Zeff) = actual pull felt by an electron after shielding.
- Shielding = reduction of nuclear attraction due to inner electrons.
- Across a period: Zeff increases, atoms get smaller.
- Down a group: shielding increases, atoms get larger.
Evaluation
- Define effective nuclear charge in your own words.
- Why is the size of chlorine smaller than sodium even though chlorine has more electrons?
- What role do inner electrons play in shielding?
Fantastic! You’ve just mastered another powerful idea that explains why elements behave the way they do. Keep reminding yourself: Chemistry is like uncovering nature’s family rules, and with Afrilearn, you are always in the front seat of discovery.
