Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s a joy to have you here today. Let me ask: have you ever boarded a Lagos BRT bus or a danfo? You know how the conductor insists that passengers must “fill the bus from the back, no empty seat in the middle!” In the same way, electrons in an atom also follow an organised way of filling available “seats” or energy levels. This organised arrangement of electrons in an atom is what we call electronic configuration.
Electronic Configurations
What is Electronic Configuration?
Electronic configuration is the arrangement of electrons in the shells, subshells, and orbitals of an atom. It shows us how electrons occupy available energy levels around the nucleus. This arrangement helps explain chemical properties, bonding, and even why elements behave the way they do.
Rules for Filling Orbitals
Just like in a bus, electrons don’t just sit anywhere—they follow rules.
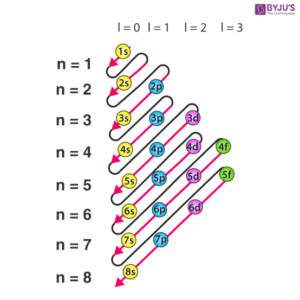
Aufbau Principle – Electrons fill orbitals starting from the lowest energy level before moving to higher ones. Imagine filling buckets: you fill the smaller one first before using the bigger one. For atoms, the order goes:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s …
Pauli Exclusion Principle – No two electrons in the same atom can have the same set of quantum numbers. In practice, this means each orbital can hold only two electrons, and they must spin in opposite directions. Think of two passengers sharing one seat: one faces left, the other right.
Hund’s Rule of Maximum Multiplicity – Electrons prefer to stay alone in an orbital until they are forced to pair up. Imagine entering an almost empty danfo bus—you’d rather sit alone until more passengers come in.
Examples of Electronic Configurations
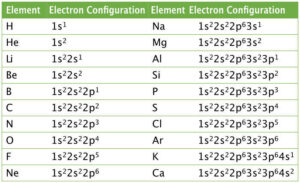
Hydrogen (Z = 1): 1s¹
Oxygen (Z = 8): 1s² 2s² 2p⁴
Sodium (Z = 11): 1s² 2s² 2p⁶ 3s¹
Notice sodium ends with 3s¹, meaning it has one electron in the outermost shell. That’s why sodium easily loses one electron to form Na⁺.
Noble Gas Notation
To shorten long configurations, we can use noble gases. For example:
Chlorine (Z = 17): [Ne] 3s² 3p⁵
Here, [Ne] represents the configuration of neon (1s² 2s² 2p⁶).
Why It Matters
Electronic configurations explain why elements in the same group of the periodic table behave alike. For example, sodium (Na: 1s² 2s² 2p⁶ 3s¹) and potassium (K: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹) both have one outer electron, so they react in similar ways.
Summary
- Electronic configuration is the orderly arrangement of electrons in an atom.
- Rules: Aufbau principle (lowest first), Pauli’s principle (max 2 electrons per orbital, opposite spins), Hund’s rule (fill singly first).
- Configurations can be written fully or in noble gas notation.
- Outer electron arrangement explains chemical behaviour.
Evaluation
- State the three main rules for writing electronic configurations.
- Write the electronic configuration of aluminium (Z = 13).
- Why do sodium and potassium have similar chemical properties?
Well done! You’ve just understood how electrons “seat themselves” in atoms. This is like the secret seating arrangement of nature, and you are now part of those who understand it. Keep going—Chemistry is opening doors of understanding that will shape your future, and Afrilearn is right here with you.
