Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my bright star! It’s always exciting to see you back, eager to learn more. Remember last time when we talked about how aromatic rings welcome certain visitors called electrophiles? Well, today we’ll see that these visits are not random at all. Aromatic compounds actually have “house rules” about where new guests are allowed to sit. Let’s uncover how these decisions are made.
Electrophilic Substitution In Aromatics II
Understanding Directing Effects
When an aromatic compound already has a group attached to it, that group can influence where the next incoming electrophile will go. This influence is called directing effect, and it determines the position of substitution on the benzene ring.
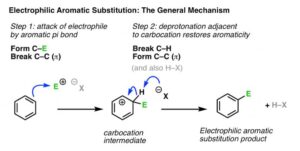
There are three main positions:
Ortho – directly next to the existing group.
Meta – one carbon away from the existing group.
Para – directly opposite the existing group.
Types of Directing Groups
Ortho/Para Directors (Activating Groups)
These groups push electron density into the ring, making certain positions (ortho and para) more attractive to electrophiles.
Examples: –OH, –NH₂, –OCH₃, alkyl groups.
Why? They have lone pairs or electron-releasing effects that stabilise the intermediate when substitution happens at ortho or para positions.
Meta Directors (Deactivating Groups)
These groups pull electron density away from the ring, making it less reactive overall, and especially unfavourable for substitution at ortho and para positions.
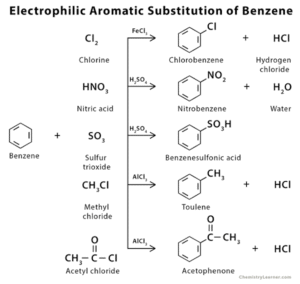
Examples: –NO₂, –SO₃H, –CN, –COOH.
Why? They are electron-withdrawing and destabilise the intermediate at ortho and para positions, making meta substitution more favourable.
Special Case – Halogens
Halogens (–Cl, –Br) are interesting because they are deactivating overall (due to electronegativity) but still direct new electrophiles to ortho and para positions because of their lone pair donation.
Think of the benzene ring as a table in a Nigerian restaurant. The group already seated is like a friend who either saves the best seats for you (ortho/para director) or blocks those seats and pushes you further away (meta director).
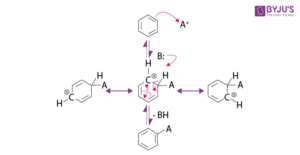
Practical Applications
Understanding directing effects is crucial when designing synthetic pathways. For example, if you want to make para-nitrophenol, you need to start with phenol (an ortho/para director) and choose reaction conditions that favour the para product.
Summary
- Directing effects guide where electrophiles attach on a substituted aromatic ring.
- Ortho/para directors are usually activating, meta directors are usually deactivating.
- Halogens are deactivating but still ortho/para directing.
Evaluation
- Define ortho, meta, and para positions in an aromatic ring.
- Give two examples each of activating and deactivating groups.
- Explain why halogens are ortho/para directing despite being deactivating.
You’re doing wonderfully! Each concept you master is making you a sharper and more strategic chemist. Afrilearn is thrilled to see you building not just knowledge, but the power to use it wisely in real-world problem-solving.
