Back to: Organic Chemistry 200 Level
Welcome to class!
Hello there, bright mind! I’m so happy you’ve shown up again, ready to learn something new. You’re doing something amazing — investing in your future! Today, we’re taking a closer look at Ethers and Epoxides. These two may look similar at first glance — both contain oxygen in between carbon atoms — but they behave in very different and interesting ways. Don’t worry, I’ll make it easy for you to understand. Let’s get started.
Ethers And Epoxides
Understanding Ethers
Ethers are organic compounds where an oxygen atom is bonded to two alkyl or aryl groups. Think of the oxygen as a tiny bridge connecting two carbon chains.
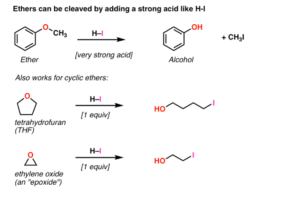
General formula: R–O–R′
Where R and R′ can be the same or different carbon groups.
Examples:
Diethyl ether (CH₃CH₂–O–CH₂CH₃): A flammable liquid once used as an anaesthetic.
Methyl tert-butyl ether (MTBE): Used in fuel to improve combustion.
Naming Ethers:
There are two common methods:
Common name: List both alkyl groups alphabetically + “ether” (e.g., ethyl methyl ether).
IUPAC name: Treat one group as an alkoxy substituent (e.g., methoxyethane).
Properties of Ethers:
Generally neutral and unreactive.
Lower boiling points than alcohols because they cannot hydrogen bond with themselves.
Good solvents in chemical reactions because they don’t react easily.
Real-life example: The smell of many perfumes and some Nigerian hair creams is partly due to compounds that include ethers.
What Are Epoxides?
Epoxides are a special type of ether, also called oxiranes. They have a three-membered ring: two carbon atoms and one oxygen atom.
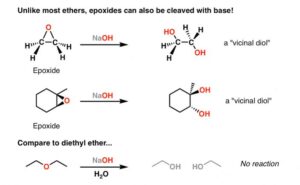
Structure: A triangle with an oxygen at the top — like a tiny, strained wheel!
Why are Epoxides Important?
The small ring is under strain, which makes epoxides more reactive than normal ethers.
They are used to make plastics, adhesives, coatings, and even pharmaceuticals.
Example:
Ethylene oxide is a common epoxide used to sterilise medical equipment.
How Do Epoxides React?
Because of the ring strain, epoxides can easily open up in the presence of acids or bases. This makes them very useful in synthesis reactions — building more complex compounds from simpler ones.
Real-life relevance: Epoxides are essential in producing epoxy resins, which are used in strong glues, paints, and coatings for electronics.
Summary
- Ethers have an oxygen bonded to two carbon groups (R–O–R′), are stable, and commonly used as solvents.
- Epoxides are cyclic ethers with a three-membered ring, and because of ring strain, they are much more reactive.
- Both are widely used in medicine, industry, and consumer products like perfumes, glues, and coatings.
Evaluation
- What is the main structural difference between ethers and epoxides?
- Why do epoxides react more easily than normal ethers?
- Write the IUPAC name for CH₃OCH₂CH₃.
- Mention one use of epoxides in daily life.
Well done! You’ve just uncovered the secrets of ethers and epoxides — two compounds that quietly play a big role in your world. The fact that you’re here learning these concepts shows how far you’re going. Stay focused and keep growing. With Afrilearn by your side, Chemistry becomes clearer and your goals more achievable. See you in the next class!
