Back to: Organic Chemistry 200 Level
Welcome to class!
Hello future chemist! Imagine a ring, but instead of all the corners being carbon atoms, one or more corners are replaced by other atoms like nitrogen, oxygen, or sulphur. That’s the special twist that gives heterocyclic compounds their unique character. From the caffeine in your coffee to the DNA in your cells, these compounds are part of your everyday life.
Heterocyclic Compounds I
What Are Heterocyclic Compounds?
Heterocyclic compounds are cyclic compounds in which the ring contains at least one atom other than carbon. This non-carbon atom is called a heteroatom.
Common heteroatoms: Nitrogen (N), Oxygen (O), and Sulphur (S).
These atoms change the chemical behaviour of the ring, making them vital in natural and synthetic compounds.
Classification of Heterocyclic Compounds
Based on Ring Size:
Three-membered rings: e.g., aziridine (contains nitrogen).
Four-membered rings: e.g., azetidine.
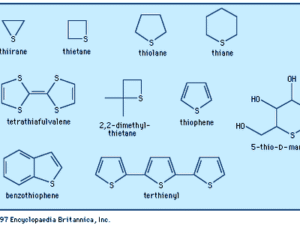
Five-membered rings: e.g., furan (O), pyrrole (N), thiophene (S).
Six-membered rings: e.g., pyridine (N).
Based on Aromaticity:
Aromatic heterocycles: Stable and follow Huckel’s rule (e.g., pyridine, furan).
Non-aromatic heterocycles: Do not have aromatic stability.
Examples in Everyday Life
Caffeine (found in coffee and tea) is a heterocyclic compound containing nitrogen.
Penicillin (an antibiotic) has a β-lactam heterocyclic ring.
DNA bases (adenine, guanine) are nitrogen-containing heterocycles.
Preparation of Heterocyclic Compounds
Furan: From dehydration of 1,4-butanediol.
Pyrrole: From heating succinimide with zinc dust.
Thiophene: From heating butane with sulphur.
Properties
Physical: Can be liquids or solids; often have distinctive odours.
Chemical: Reactivity depends on the heteroatom; nitrogen heterocycles are often basic, while oxygen-containing ones are less reactive towards acids.
Many aromatic heterocycles participate in electrophilic substitution like benzene.
Importance in Life and Industry
Medicines: Many drugs contain heterocyclic rings.
Agriculture: Found in pesticides and herbicides.
Biochemistry: Essential in genetic material, vitamins, and enzymes.
Summary
- Heterocyclic compounds contain at least one non-carbon atom in their ring.
- Classified by ring size and aromaticity.
- Found in nature (DNA, caffeine) and in synthetic products (drugs, pesticides).
Evaluation
- Define heterocyclic compounds and give two examples.
- Classify pyridine and thiophene based on aromaticity and ring size.
- Name one heterocyclic compound found in DNA.
- Give one industrial use of heterocyclic compounds.
Great work! You’ve just unlocked the door to understanding some of the most important structures in nature and medicine. With Afrilearn, every topic takes you closer to mastering the language of life itself — chemistry.
