Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m glad to have you here today. Imagine cooking a pot of jollof rice. The grains of rice stick together in a way that makes the food delicious. But the stickiness isn’t too strong—it’s just enough to hold them close while still being easy to eat. In Chemistry, there’s something similar called hydrogen bonding, a special type of attraction between molecules that makes them “stick” to each other in unique ways. Today, we will uncover why this bond is so important in everyday life.
Hydrogen Bonding
What is Hydrogen Bonding?
Hydrogen bonding is a type of intermolecular force that occurs when a hydrogen atom is covalently bonded to a very electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F), and this hydrogen is attracted to a lone pair of electrons on another electronegative atom.
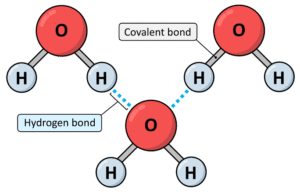
Think of hydrogen as a child holding the hand of one parent (oxygen, nitrogen, or fluorine) but reaching out with the other hand to grab onto another parent nearby. That “reach” is the hydrogen bond.
Conditions for Hydrogen Bonding
The molecule must contain a hydrogen atom bonded to a highly electronegative atom (N, O, or F).
The electronegative atom must have lone pairs of electrons.
The bond is not as strong as a covalent bond, but it is stronger than van der Waals forces.
Types of Hydrogen Bonding
Intermolecular Hydrogen Bonding – occurs between different molecules.
Example: In water (H₂O), each molecule can form hydrogen bonds with neighbouring molecules, making water liquid at room temperature rather than a gas.
Intramolecular Hydrogen Bonding – occurs within the same molecule.
Example: In o-nitrophenol, hydrogen bonding happens inside the molecule, leading to differences in boiling point compared to p-nitrophenol.
Importance of Hydrogen Bonding in Everyday Life
Water’s Properties: Water has an unusually high boiling point because of hydrogen bonding. This is why rivers and lakes don’t just evaporate quickly under the hot Nigerian sun.
Ice Structure: Ice floats on water because hydrogen bonding arranges water molecules in a way that makes ice less dense. Without this, rivers and lakes would freeze from the bottom up, making life in water impossible.
Proteins and DNA: The shape of proteins and the double helix structure of DNA in our bodies depend heavily on hydrogen bonds. Without them, life as we know it would not exist.
Everyday Substances: Hydrogen bonding affects solubility and boiling points. For example, alcohols dissolve well in water due to hydrogen bonding.
Summary
- Hydrogen bonding is a special attraction involving hydrogen and electronegative atoms (N, O, F).
- It can be intermolecular (between molecules) or intramolecular (within one molecule).
- It explains water’s high boiling point, why ice floats, and the stability of biological molecules like proteins and DNA.
Evaluation
- Define hydrogen bonding in your own words.
- Give two everyday examples of substances where hydrogen bonding plays a role.
- Differentiate between intermolecular and intramolecular hydrogen bonding with examples.
- Why does ice float on water?
You did excellently today! Remember, each small concept you master is like building a powerful toolkit for understanding the world around you. Keep learning with Afrilearn—you are unstoppable.
