Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m glad you’re here today. Let’s start with a simple picture. Imagine two friends—one has extra meat in his plate of jollof rice, while the other has none. Out of generosity, the one with extra gives a piece to the other, making both satisfied. In Chemistry, a very similar thing happens when one atom has “extra” electrons that it can give away, while another atom needs electrons to become stable. This transfer of electrons from one atom to another is what we call ionic bonding.
Ionic Bonding
What is Ionic Bonding?
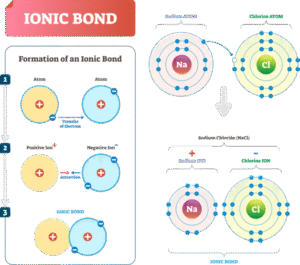
Ionic bonding is the electrostatic force of attraction between positively charged ions (cations) and negatively charged ions (anions), formed when electrons are transferred from one atom to another.
Metals usually lose electrons to form cations.
Non-metals usually gain electrons to form anions.
How Ionic Bonding Works
Electron Transfer:
A metal atom donates one or more of its outer (valence) electrons to a non-metal atom.
The metal becomes a positively charged ion, and the non-metal becomes a negatively charged ion.
Electrostatic Attraction:
Opposite charges attract strongly, and this attraction forms the ionic bond.
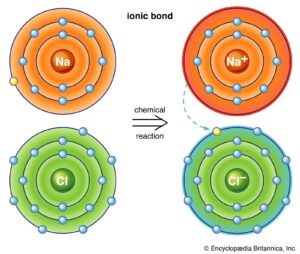
Example: Sodium Chloride (NaCl)
Sodium (Na) has 1 electron in its outer shell.
Chlorine (Cl) has 7 electrons in its outer shell and needs 1 more to complete the octet.
Sodium transfers its 1 electron to chlorine.
Sodium becomes Na⁺, chlorine becomes Cl⁻.
The strong attraction between Na⁺ and Cl⁻ forms NaCl, common table salt.
Properties of Ionic Compounds
High melting and boiling points: Strong electrostatic forces hold the ions together, so much heat is needed to separate them.
Conduct electricity in molten or aqueous state: Ions are free to move and carry charge.
Brittle: They can shatter when hit because ions of like charges are forced close together, causing repulsion.
Soluble in water: Many ionic compounds dissolve easily in water (e.g., NaCl).
Other Examples of Ionic Compounds
Magnesium oxide (MgO): Mg²⁺ and O²⁻.
Calcium fluoride (CaF₂): Ca²⁺ and F⁻.
Everyday Connection
The salt you sprinkle on food is sodium chloride—an ionic compound!
Toothpaste often contains fluoride (like sodium fluoride, NaF), which protects teeth.
Fertilisers used by farmers (like potassium chloride, KCl) are ionic compounds that help crops grow.
Summary
- Ionic bonding = transfer of electrons from metals to non-metals.
- Metals form cations; non-metals form anions.
- The attraction between oppositely charged ions creates strong bonds.
- Ionic compounds are usually crystalline solids with high melting points, soluble in water, and conductors when molten or in solution.
Evaluation
- Define ionic bonding.
- Explain, using a diagram if possible, how sodium and chlorine form sodium chloride.
- State two physical properties of ionic compounds.
Excellent job! Today you’ve seen how atoms “share gifts” to achieve stability, just like generous friends. With Afrilearn, Chemistry becomes simple, practical, and connected to your everyday life. Keep going—you’re building the foundation for mastery!
