Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s wonderful to have you here again. Let’s start with something you can picture. Imagine building a house with blocks. The stronger and tighter the blocks fit together, the sturdier the house becomes. In Chemistry, when ions (the “blocks”) come together to form a solid ionic compound, the strength of attraction that holds them tightly is called the lattice energy. The “blueprint” that helps us calculate and understand this energy is known as the Born–Haber cycle.
Lattice Energy (Born-haber Cycle)
What is Lattice Energy?
Lattice energy is the energy released when one mole of an ionic solid is formed from its gaseous ions.
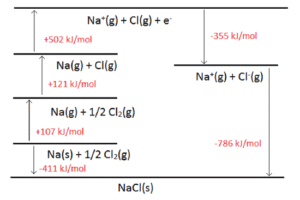
Example: When Na⁺(g) and Cl⁻(g) combine to form NaCl(s), energy is released.
The stronger the attraction between the ions, the higher the lattice energy.
Factors Affecting Lattice Energy
Charge of ions: The higher the charge, the stronger the attraction, and the larger the lattice energy. (e.g., MgO > NaCl).
Size of ions: Smaller ions attract more strongly, so compounds with small ions have larger lattice energy.
Arrangement of ions in the lattice: Some crystal structures allow stronger packing and hence higher lattice energy.
What is the Born–Haber Cycle?
The Born–Haber cycle is a step-by-step thermochemical process used to calculate the lattice energy of an ionic compound. It shows all the energy changes involved in forming an ionic solid from its elements.
Steps in the Born–Haber Cycle for NaCl:
Atomisation: Na(s) → Na(g). Energy is needed.
Ionisation energy: Na(g) → Na⁺(g) + e⁻. Energy is needed to remove an electron.
Dissociation of chlorine molecule: ½Cl₂(g) → Cl(g). Energy is needed to break the bond.
Electron affinity: Cl(g) + e⁻ → Cl⁻(g). Energy is released.
Formation of solid: Na⁺(g) + Cl⁻(g) → NaCl(s). Energy released = lattice energy.
By applying Hess’s Law (energy is conserved in a cycle), we can calculate the lattice energy indirectly.
Why is the Born–Haber Cycle Important?
It helps compare the stability of different ionic compounds.
It explains why some compounds form easily while others do not.
It helps predict solubility and hardness of ionic compounds.
Everyday Connection
The salt (NaCl) you eat daily is stable because of high lattice energy.
Hard materials like MgO (used in refractory bricks) owe their strength to very high lattice energy.
Fertilisers such as KCl remain solid until dissolved because of the stability provided by their ionic lattice.
Summary
- Lattice energy = energy released when gaseous ions form a solid crystal.
- Stronger charges and smaller ion sizes → higher lattice energy.
- The Born–Haber cycle breaks down the formation of an ionic compound into steps: atomisation, ionisation, dissociation, electron affinity, and lattice formation.
- It explains stability and properties of ionic solids.
Evaluation
- Define lattice energy.
- State two factors that affect lattice energy.
- Write the steps in the Born–Haber cycle for the formation of sodium chloride.
Well done! You’ve just seen how ionic compounds are like sturdy houses built from strong “blocks” of ions. With Afrilearn, you’re mastering Chemistry step by step—be proud of your progress. The next time you see table salt or tough materials like MgO, remember: their strength comes from lattice energy!
