Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always a joy to have you here. Let me start with something simple. Picture two friends at school: one is generous and always shares his pens, while the other prefers to hold onto her own. In Chemistry, some elements behave like the generous friend—they easily give away electrons. Others are like the one who holds tightly to what they have. The first group represents metals, while the second represents non-metals. The differences in their properties are what we call metallic and non-metallic properties.
Metallic And Non-metallic Properties
What are Metallic Properties?
Metallic properties are the characteristics that metals display due to their ability to lose electrons and form positive ions (cations).
Some key metallic properties include:
Good conductors of heat and electricity (like copper wires in our homes).
Malleable and ductile (they can be hammered into sheets or drawn into wires).
Lustrous (shiny surface, like aluminium foil).
Tend to lose electrons easily, forming cations (Na → Na⁺ + e⁻).
What are Non-Metallic Properties?
Non-metallic properties are the characteristics that non-metals display due to their tendency to gain electrons and form negative ions (anions).
Some key non-metallic properties include:
Poor conductors of heat and electricity (like plastic or sulphur).
Brittle (break easily when hammered, unlike metals).
Dull appearance (not shiny).
Tend to gain electrons easily, forming anions (Cl + e⁻ → Cl⁻).
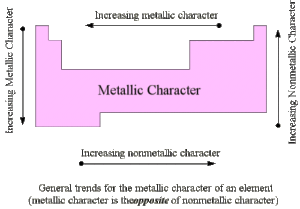
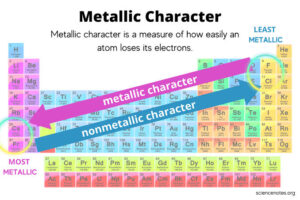
Trends in the Periodic Table
Across a Period (left to right):
Metallic properties decrease. Why? Atoms hold on more tightly to their electrons due to increased nuclear charge.
Non-metallic properties increase. Why? Atoms have higher electronegativity and electron affinity, so they prefer to gain electrons.
Example: Sodium (metallic) → Magnesium → Aluminium → Silicon → Phosphorus → Sulphur → Chlorine (non-metallic).
Down a Group (top to bottom):
Metallic properties increase. Why? Atoms get larger, so they lose electrons more easily.
Non-metallic properties decrease. Why? The nuclear pull on added electrons weakens as atoms get larger.
Example: In Group 7 (halogens), fluorine is the most non-metallic, while iodine is less so.
Everyday Examples
Cooking pots are made of aluminium or stainless steel (metals) because they conduct heat well.
Electrical cables use copper because it conducts electricity efficiently.
Non-metals like chlorine are used in disinfectants because of their high reactivity.
Graphite, a non-metal, is used in pencils, showing how diverse non-metals can be.
Summary
- Metals lose electrons easily; non-metals gain electrons.
- Metallic properties: conductivity, malleability, lustre, electron loss.
- Non-metallic properties: poor conductivity, brittleness, dullness, electron gain.
- Across a period: metallic character decreases, non-metallic character increases.
- Down a group: metallic character increases, non-metallic character decreases.
Evaluation
- State two metallic properties and two non-metallic properties.
- Why does metallic character increase down a group?
- Arrange these elements in order of increasing metallic character: Sodium (Na), Aluminium (Al), Sulphur (S).
Well done! You’ve just understood why some elements act like generous givers (metals) while others prefer to hold on (non-metals). With Afrilearn, Chemistry becomes as real as the objects around you. Keep your curiosity alive—you are building the skills of a future innovator!
