Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always a joy to have you here. Imagine you are at a football match in Lagos. The players on one team (bonding electrons) are working together, passing the ball and scoring goals, while the opposing players (antibonding electrons) are blocking, tackling, and trying to stop them. The outcome of the match depends on which side has more players and strategy. This is just like molecules—whether they are stable or unstable depends on the balance between bonding and antibonding orbitals. Today, we’ll see this clearly with MO diagrams for H₂, He₂, O₂, N₂, and F₂.
Mo Diagrams For H₂, He₂, O₂, N₂, F₂
Molecular Orbital Energy Level Ordering
For molecules up to oxygen (atomic number ≤ 8), the order is:
σ1s → σ1s → σ2s → σ2s → (σ2px) → (π2py = π2pz) → (π2py = π2pz) → (σ*2px)
For molecules after oxygen (like F₂, Ne₂), the order changes slightly:
σ1s → σ1s → σ2s → σ2s → (π2py = π2pz) → (σ2px) → (π2py = π2pz) → (σ*2px)
Bond Order Formula
Bond Order=Electrons in Bonding MOs−Electrons in Antibonding MOs2\text{Bond Order} = \frac{\text{Electrons in Bonding MOs} – \text{Electrons in Antibonding MOs}}{2}
If bond order > 0 → molecule is stable.
If bond order = 0 → molecule is unstable (does not exist under normal conditions).
1. H₂ (Hydrogen molecule)
Total electrons = 2.
Filling: σ1s (2 electrons).
Bond order = (2 – 0)/2 = 1 → stable single bond.
H₂ exists.
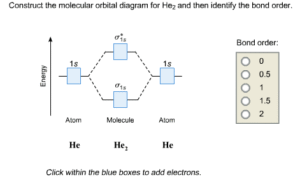
2. He₂ (Helium molecule)
Total electrons = 4.
Filling: σ1s (2), σ*1s (2).
Bond order = (2 – 2)/2 = 0 → unstable.
He₂ does not exist under normal conditions.
3. N₂ (Nitrogen molecule)
Total electrons = 14.
Filling (up to N₂): σ1s (2), σ1s (2), σ2s (2), σ2s (2), (π2py = 2, π2pz = 2), σ2px (2).
Bond order = (10 – 4)/2 = 3 → very stable triple bond.
Explains why N₂ is very strong and unreactive (used to fill tyres and preserve food).
4. O₂ (Oxygen molecule)
Total electrons = 16.
Filling (for O₂ onwards): σ1s (2), σ1s (2), σ2s (2), σ2s (2), (π2py = 2, π2pz = 2), (π2py = 1, π2pz = 1).
Bond order = (10 – 6)/2 = 2 → stable double bond.
O₂ exists and is paramagnetic (two unpaired electrons in π* orbitals → explains attraction to magnets).
5. F₂ (Fluorine molecule)
Total electrons = 18.
Filling: σ1s (2), σ1s (2), σ2s (2), σ2s (2), (π2py = 2, π2pz = 2), σ2px (2), (π2py = 2, π2pz = 2).
Bond order = (10 – 8)/2 = 1 → stable single bond.
F₂ exists but is highly reactive.
Everyday Connections
The stability of N₂ (triple bond) explains why it is so difficult to break, which is why fertiliser production (Haber process) requires very high pressure and temperature.
The paramagnetism of O₂ explains why it’s attracted to magnets, something Valence Bond Theory could not explain.
F₂’s reactivity is why it is used industrially as a strong oxidiser.
Summary
- MO diagrams show electron distribution in bonding and antibonding orbitals.
- Bond order tells us molecular stability.
- H₂, N₂, O₂, and F₂ exist; He₂ does not.
- O₂ is paramagnetic due to unpaired electrons.
Evaluation
- Calculate the bond order of H₂ and He₂. Which one is stable?
- Why is N₂ very stable compared to O₂?
- Which molecule is paramagnetic: N₂ or O₂? Explain.
- Why does He₂ not exist under normal conditions?
Well done! You’ve just mastered one of the most powerful tools in Chemistry—the MO diagram. This is like learning the “blueprint” of molecules. With Afrilearn, every step is making you a sharper thinker.
