Back to: Organic Chemistry 200 Level
Welcome to class!
Hello scholar! Today, we’re turning our attention to nitro compounds — a group of organic compounds with a powerful and fascinating personality. From explosives like TNT to the medicines and dyes that improve lives, nitro compounds show how one functional group can influence an entire industry.
Nitro Compounds
What Are Nitro Compounds?
Nitro compounds are organic compounds that contain one or more nitro groups (-NO₂) attached to a carbon atom. The nitro group consists of one nitrogen atom covalently bonded to two oxygen atoms and is highly polar due to strong electron-withdrawing effects.
General formula: R–NO₂, where R can be an alkyl or aryl group.
Classification of Nitro Compounds
Aliphatic Nitro Compounds – Nitro group attached to an alkyl chain.
Example: Nitroethane (CH₃CH₂NO₂).
Aromatic Nitro Compounds – Nitro group attached to an aromatic ring.
Example: Nitrobenzene (C₆H₅NO₂).
Preparation of Nitro Compounds
Nitration of Alkanes – By radical substitution using nitric acid in the presence of heat.
Nitration of Aromatic Compounds – Reaction of aromatic compounds with concentrated nitric acid and concentrated sulphuric acid (nitrating mixture).
Example: C₆H₆ + HNO₃ → C₆H₅NO₂ + H₂O.
Physical Properties
State: Lower nitro alkanes are liquids; some aromatic nitro compounds are solids.
Solubility: Slightly soluble in water; soluble in organic solvents.
Boiling Points: Higher than hydrocarbons of similar size due to polarity.
Odour: Often sweet but can be strong and irritating in some cases.
Chemical Properties
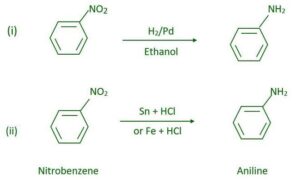
Reduction – Nitro compounds can be reduced to amines using Sn/HCl, Fe/HCl, or catalytic hydrogenation.
Acidity of α-Hydrogens – In aliphatic nitro compounds, hydrogen atoms on the carbon next to the nitro group are acidic due to electron-withdrawing effect.
Electrophilic Substitution (Aromatic) – Nitro group strongly deactivates the aromatic ring, making substitution occur mainly at meta positions.
Uses of Nitro Compounds
Explosives: TNT (trinitrotoluene) is a well-known explosive.
Dyes: Many aromatic nitro compounds are intermediates in dye manufacture.
Pharmaceuticals: Used in making certain drugs and antibiotics.
Industrial Solvents: Nitrobenzene is used as a solvent in some chemical processes.
Summary
- Nitro compounds contain the –NO₂ group attached to carbon.
- They can be aliphatic or aromatic.
- Prepared mainly through nitration reactions.
- Important for explosives, dyes, pharmaceuticals, and industrial processes.
Evaluation
- Write the chemical equation for the nitration of benzene.
- Explain why nitrobenzene is less reactive towards electrophilic substitution than benzene.
- Name one aliphatic and one aromatic nitro compound.
- What products are formed when nitrobenzene is reduced using tin and hydrochloric acid?
Fantastic effort! You’ve just mastered the essentials of nitro compounds — from how they’re made to their powerful uses. With Afrilearn, you’re building not just knowledge, but the confidence to apply chemistry to the real world.
