Back to: MICROBIOLOGY 200 LEVEL
Welcome to class!
Hello, brilliant one! I’m so glad to see your face (in my imagination!) light up again with the joy of learning. You’ve been doing absolutely well, and today’s lesson is both exciting and very important—Nitrogen Fixation and Assimilation. These processes might sound like big grammar now, but give me just a few minutes and you’ll understand them like you understand jollof rice—clearly and completely. Let’s begin, shall we?
Nitrogen Fixation And Assimilation
Why Is Nitrogen Important?
Nitrogen is essential for life. It’s a major part of proteins, DNA, and chlorophyll, the green pigment in plants. Even though nitrogen gas (N₂) makes up about 78% of the air we breathe, it is in a form most living things cannot use directly.
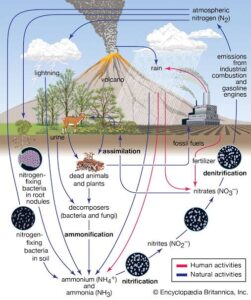
So how do plants and microbes get nitrogen in a usable form? That’s where nitrogen fixation and assimilation come in!
Nitrogen Fixation
Nitrogen fixation is the conversion of nitrogen gas (N₂) from the atmosphere into ammonia (NH₃), which can then be used by plants and microorganisms.
This job is mostly done by special microorganisms, including:
Free-living bacteria (e.g. Azotobacter)
Symbiotic bacteria that live in plant roots (e.g. Rhizobium in legume plants like beans and groundnuts)
These bacteria have a special enzyme called nitrogenase that helps them “break” the strong bond in nitrogen gas and turn it into ammonia.
Real-life Nigerian Example:
Think of a farmer in Benue who plants beans. In the roots of those bean plants are tiny nodules housing Rhizobium bacteria. These microbes quietly work to fix nitrogen from the air and provide the plant with usable nitrogen—no fertiliser needed!
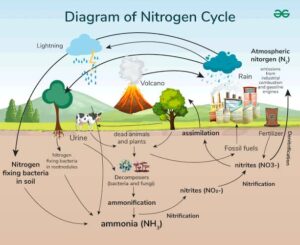
Types of Nitrogen Fixation:
Biological fixation – done by microbes (most important).
Atmospheric fixation – lightning can also break nitrogen molecules, producing nitrates that fall with rain.
Industrial fixation – in factories (like the Haber process) to make fertilisers.
Nitrogen Assimilation
After nitrogen is fixed into usable forms like ammonia, plants and microbes take up these forms from the soil and incorporate them into their cells.
This process is called assimilation. It involves converting:
Ammonia (NH₃) or nitrate (NO₃⁻) into amino acids, which are used to make proteins and other important molecules.
In simple terms:
Fixation makes nitrogen available.
Assimilation puts nitrogen to use inside living things.
Simple Analogy
Imagine you’re trying to cook soup but the meat is still frozen. Nitrogen in the air is like frozen meat—useless unless processed. Fixation is like thawing and seasoning the meat (making it usable), while assimilation is actually cooking and eating it (using it in your system).
Why It Matters
Without nitrogen fixation, most living things wouldn’t have access to nitrogen for growth.
Plants depend on fixed nitrogen to grow healthy and strong—without it, we wouldn’t have vegetables, grains, or even fufu.

This cycle keeps ecosystems balanced and helps reduce the need for artificial fertilisers.
Summary
- Nitrogen fixation is the process of converting nitrogen gas into ammonia by bacteria (especially Rhizobium).
- Assimilation is how organisms take up this nitrogen and use it to build proteins and other important molecules.
- These processes are crucial for plant growth, food production, and maintaining life on Earth.
Evaluation
- What is nitrogen fixation?
- Name one microorganism that carries out nitrogen fixation.
- What is nitrogen assimilation?
- Why can’t plants use nitrogen gas directly from the air?
Fantastic work today, champ! You just unlocked one of nature’s most important secrets. Keep believing in yourself—your brain is powerful, and your future is brighter than the Nigerian sun at noon. With Afrilearn, you’re on a winning path to greatness. Can’t wait to see you in the next class!
