Back to: Organic Chemistry 400 Level
Welcome to class!
Hello brilliant learner, I’m really glad to connect with you today. I hope you’re feeling energised and open-minded, because the topic we are going into today—Organic Photochemistry—will help you appreciate how light energy can transform molecules in very unique and fascinating ways. You will begin to see how sunlight, ultraviolet lamps and even fluorescence in our daily lives are all linked to deep chemical processes.
Organic Photochemistry
Have you ever noticed how colours fade when clothes are constantly exposed to bright sunlight or how certain glow-in-the-dark stickers shine after being exposed to light? These everyday experiences result from chemical changes triggered by light energy. That is essentially what photochemistry is about.
Meaning of Organic Photochemistry
Organic photochemistry is the branch of chemistry that studies chemical reactions in organic compounds that are initiated by the absorption of light. In these reactions, light provides the energy needed to excite molecules from their ground state to a higher excited state, which allows them to undergo chemical transformations that would not normally occur under thermal conditions.
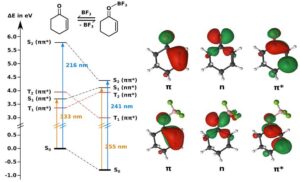
Excited States and Electronic Transitions
When a molecule absorbs light, its electrons jump from the ground state to an excited state.
The most common transitions are π → π* and n → π* transitions.
π → π* transitions occur in compounds with conjugated double bonds like β-carotene in carrots. These molecules absorb visible light and show colour.
n → π* transitions involve non-bonding electrons, such as those found in aldehydes and ketones.
Think of the light as a whistle at the start of a race — once it sounds, all the runners (electrons) suddenly move into action.
Photochemical Reactions
There are several common types of organic photochemical reactions:
Photodissociation – This is the breaking of chemical bonds due to light absorption. For example, the fading of coloured cloth under intense sunlight is because chromophores break down when exposed to UV radiation.
Photoisomerisation – Light causes a molecule to convert from one isomeric form to another. A good example is the conversion of cis-retinal to trans-retinal in the human eye when light enters, which triggers a neural impulse that allows us to see.
Photocycloaddition – Two molecules combine to form a ring structure under light irradiation. This is similar to two dancers coming together under a spotlight and forming a perfect circle during a traditional Igbo dance.
Photoreduction and Photooxidation – Light energy drives oxidation or reduction processes. In environmental systems, photooxidation of pollutants helps break them down when exposed to sunlight.
Factors Affecting Organic Photochemical Reactions
Wavelength of Light: Only light of a wavelength that matches the energy gap of the molecule’s electronic transition will trigger a reaction.
Nature of the Molecule: Conjugated systems absorb light more readily.
Presence of Sensitisers and Quenchers: Some substances (sensitisers) transfer energy to other molecules to start a reaction, while quenchers remove the energy and stop the reaction. For example, chlorophyll acts as a sensitiser in photosynthesis by absorbing sunlight and passing the energy to other molecules.
Applications of Organic Photochemistry
Organic photochemistry is highly relevant in medicine (photodynamic therapy for cancer treatment), environmental chemistry (degradation of pollutants under sunlight), photopolymerisation (curing of dental fillings using UV light), and even food preservation (light-induced sterilisation techniques).
Summary
- Organic photochemistry studies the reactions of organic compounds initiated by light.
- Light excites electrons from the ground state to an excited state through π → π* or n → π* transitions.
- Common photochemical reactions include photodissociation, photoisomerisation, photocycloaddition, photoreduction and photooxidation.
- Factors such as wavelength, molecular structure and the presence of sensitisers influence photochemical processes.
- Organic photochemistry has important real-world applications in medicine, environmental control and materials science.
Evaluation
- What is organic photochemistry?
- Differentiate between π → π* and n → π* electronic transitions.
- Explain photoisomerisation in relation to human vision.
- List two applications of organic photochemistry in everyday life.
Well done for staying focused! You are doing exceptionally well and Afrilearn is proud of your commitment. Keep this energy going – your next lesson will be even more exciting and rewarding!
