Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my brilliant learner! I’m glad to see you again as we continue our journey into Pericyclic Reactions. In the first part, we learnt the basics and main types — cycloaddition, electrocyclic reactions, and sigmatropic rearrangements. Today, we will go deeper, looking at how to predict the outcomes of these reactions using orbital symmetry concepts, and how real-life examples make these reactions powerful tools in organic synthesis.
Pericyclic Reactions II
Cycloaddition Reactions in Detail
In a cycloaddition, two or more π systems join to form a ring. The most common example, the Diels–Alder reaction, is a [4+2] cycloaddition. This means four π electrons from a diene combine with two π electrons from a dienophile. The reaction is stereospecific — if the dienophile has substituents on the same side, they remain on the same side in the product. Cycloadditions can also be photochemical, such as the [2+2] cycloaddition, where two alkenes form a cyclobutane under UV light.
Electrocyclic Reactions in Detail
Electrocyclic reactions involve the opening or closing of a conjugated π system into a ring, or the reverse. For example, a conjugated triene can cyclise into a cyclohexadiene. The way the substituents rotate during the reaction (conrotatory or disrotatory motion) depends on whether the reaction is thermal or photochemical. Under thermal conditions, a 4n+2 π-electron system undergoes disrotatory closure, while a 4n π-electron system undergoes conrotatory closure. Light reverses this pattern.
Sigmatropic Rearrangements
In a sigmatropic rearrangement, a sigma bond shifts to a new position while π bonds reorganise. A common example is the Cope rearrangement, where a 1,5-diene rearranges to another 1,5-diene. Another is the Claisen rearrangement, where an allyl vinyl ether rearranges to an unsaturated carbonyl compound. These reactions occur in a single step with no intermediates and are guided by the same orbital symmetry principles.
Woodward–Hoffmann Rules in Practice
These rules state that pericyclic reactions are “allowed” or “forbidden” based on conservation of orbital symmetry. For a reaction to proceed easily, the symmetry of the interacting molecular orbitals must match in the transition state. This determines whether a reaction happens under heat or light, and whether motion is conrotatory or disrotatory.
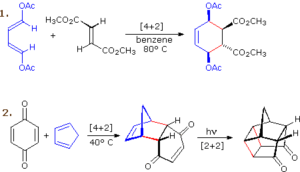
Applications in Real Life
In pharmaceutical synthesis, pericyclic reactions are used to create complex ring systems with precise stereochemistry — for example, in anti-inflammatory drugs and antibiotics. In agriculture, they are applied to develop crop protection chemicals. In natural processes, sunlight triggers a photochemical electrocyclic reaction to convert 7-dehydrocholesterol in the skin into vitamin D₃.
Summary
- Cycloadditions join two or more π systems to form a ring, e.g., the [4+2] Diels–Alder reaction.
- Electrocyclic reactions open or close a π system into a ring, with conrotatory or disrotatory motion depending on electron count and heat/light conditions.
- Sigmatropic rearrangements shift a sigma bond and reorganise π bonds, e.g., Cope and Claisen rearrangements.
- Woodward–Hoffmann rules predict whether a reaction is thermally or photochemically allowed.
- Pericyclic reactions are valuable in drug synthesis, agriculture, and natural processes like vitamin D formation.
Evaluation
- What is the difference between a [4+2] and [2+2] cycloaddition?
- Under thermal conditions, how does a 4n+2 π-electron system close in an electrocyclic reaction?
- Give one example each of a Cope and a Claisen rearrangement.
- What do the Woodward–Hoffmann rules predict?
- Mention one industrial and one natural application of pericyclic reactions.
You are not just learning organic chemistry — you are learning how nature itself builds molecules with elegance and precision. Afrilearn celebrates your growing expertise, and your ability to understand orbital symmetry means you’re thinking like a true chemist.
