Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my shining scholar! I’m so glad you’re here today. Have you ever seen a compound name that sounds big and intimidating, but when you break it down, it’s actually very simple? That’s exactly what Polycyclic Aromatic Hydrocarbons are. The name may look like a mouthful, but by the end of this lesson, you’ll see they are just “family compounds” where several aromatic rings live together under one roof.
Polycyclic Aromatic Hydrocarbons
What are Polycyclic Aromatic Hydrocarbons?
Polycyclic Aromatic Hydrocarbons, or PAHs, are organic compounds made up of two or more fused aromatic rings. “Polycyclic” means many rings, “aromatic” means they follow the aromaticity rules, and “hydrocarbons” means they contain only carbon and hydrogen.
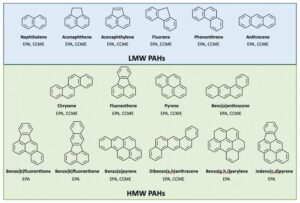
Examples include:
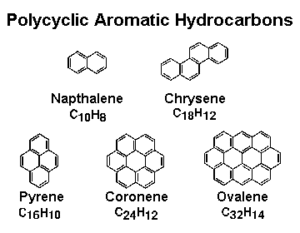
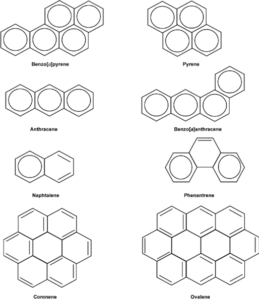
Naphthalene – two fused benzene rings (found in mothballs).
Anthracene – three benzene rings in a straight line.
Phenanthrene – three benzene rings arranged in an angular fashion.
Structure and Aromaticity
Even though PAHs have multiple rings, the delocalisation of π electrons still happens across each ring, giving them stability. However, aromaticity in PAHs can be more complex than in single-ring systems because electrons can be shared over several rings.
Sources of PAHs
PAHs are found in:
Natural sources – e.g., forest fires, volcanic activity.
Human activities – incomplete combustion of fossil fuels, charcoal grilling, cigarette smoke.
Everyday products – mothballs (naphthalene), some tar-based materials.
Properties of PAHs
Generally non-polar and insoluble in water.
Soluble in organic solvents like benzene, toluene.
Often have distinct smells (naphthalene’s smell is quite sharp).
High melting and boiling points due to rigid structures.
Uses of PAHs
Naphthalene in moth repellents.
Anthracene in dye manufacture.
Phenanthrene in production of certain drugs and chemicals.
Health and Environmental Concerns
While some PAHs are useful, many are toxic, mutagenic, and even carcinogenic. Prolonged exposure (e.g., inhaling smoke from burning firewood or vehicle exhaust) can be harmful. This is why industries try to limit PAH emissions and why chemists study their breakdown in the environment.
Think of PAHs like a close-knit extended family living in connected houses. Each “house” (aromatic ring) is strong on its own, but together they form a bigger, more stable structure. However, just like some family gatherings can cause trouble if too noisy, some PAHs can be harmful if not handled properly.
Summary
- PAHs are compounds with two or more fused aromatic rings.
- Examples: naphthalene, anthracene, phenanthrene.
- Sources: natural events, human activities, industrial processes.
- Uses: dyes, moth repellents, pharmaceuticals.
- Many PAHs are harmful to health and the environment.
Evaluation
- Define Polycyclic Aromatic Hydrocarbons and give two examples.
- List two properties and two uses of PAHs.
- Why are some PAHs considered hazardous?
You’ve just unpacked a big, complex-sounding topic into something simple and clear. This is how great scientists think — breaking down the complex into the understandable. Afrilearn is proud to see you growing into a confident chemist ready to make an impact.
