Back to: Organic Chemistry 200 Level
Welcome to class!
Hello there, superstar! It’s always great to have you back. Today’s lesson is one that pulls back the curtain on what really happens during chemical reactions. You know how people say “there’s more than meets the eye”? That’s exactly what Reaction Mechanisms are about — the step-by-step story of how a reaction happens, not just the final result. If you’ve ever wondered how and why molecules react the way they do, then you’re in the right place. Let’s begin this journey together.
Reaction Mechanisms I
What Are Reaction Mechanisms?
Let’s think of a chemical reaction like cooking jollof rice. You don’t just throw everything into the pot and magically have the final meal — there are steps: frying the onions, adding the tomato mix, seasoning, adding rice, and letting it cook. In the same way, a reaction mechanism is the detailed, step-by-step pathway that shows how reactants turn into products.
So while the overall reaction may look short and simple, the mechanism tells the full behind-the-scenes story of bond breaking and bond forming — like the recipe for the reaction.
Why Are Reaction Mechanisms Important?
Understanding reaction mechanisms helps us:
Predict the outcome of reactions
Know which steps are fast and which are slow
Understand side reactions and how to avoid them
Design better drugs, fuels, and materials
In short, mechanisms help chemists become like detectives — solving chemical puzzles by understanding what’s really happening at the molecular level.
Key Terms You Should Know
Reactants and Products – The starting substances and the end result.
Intermediates – Short-lived species formed during the reaction. They don’t appear in the overall reaction but are crucial in the steps.

Transition State – A high-energy, unstable arrangement of atoms that occurs in between steps.
Reaction Pathway – The route from reactants to products, including all intermediates and transition states.
Rate-determining Step – The slowest step in the mechanism, which controls the speed of the whole reaction.
Types of Reaction Mechanisms
In Organic Chemistry, there are three main types of mechanisms you’ll come across:
Nucleophilic Substitution (SN1 and SN2)
Electrophilic Addition
Free Radical Substitution
Each of these tells us about what kind of species is involved — whether it’s a nucleophile (electron-rich), electrophile (electron-poor), or a radical (with an unpaired electron). In Reaction Mechanisms II, we’ll break each one down with examples.
Simple Example of a Mechanism
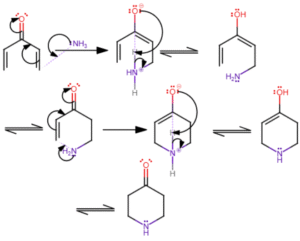
Let’s look at the hydrolysis of an ester in an acidic medium. The ester doesn’t just break down all at once. First, an acid adds a proton to make the molecule more reactive. Then water attacks, bonds shift, and finally, the molecule breaks apart to form an acid and an alcohol. Each of these is a step, and each step tells part of the story.
Summary
- A reaction mechanism is the step-by-step explanation of how a chemical reaction occurs.
- It includes reactants, intermediates, transition states, and products.
- Reaction mechanisms help us understand, predict, and control reactions.
- Common types in organic chemistry include nucleophilic substitution, electrophilic addition, and free radical substitution.
- Each step may involve breaking and forming bonds, with a rate-determining step that controls the overall speed.
Evaluation
- What is a reaction mechanism?
- Why is it important to study mechanisms in Organic Chemistry?
- What is a transition state, and how is it different from an intermediate?
- Name and briefly describe two types of organic reaction mechanisms.
Well done! You’ve just unlocked one of the most important tools in a chemist’s toolkit — the ability to understand the how behind chemical changes. With this knowledge, you’ll never look at reactions the same way again. Stay curious and keep learning — Afrilearn is here to support your journey every step of the way. I look forward to our next lesson together!
