Back to: Organic Chemistry 400 Level
Welcome to class!
Hello brilliant learner, it’s wonderful to have you here again today. I hope you’re feeling curious and motivated because what we’re about to learn forms the backbone of advanced Organic Chemistry. Whether you’re dreaming of becoming a medical researcher, industrial chemist or academic scholar, understanding Reaction Mechanisms will give you a solid foundation.
Reaction Mechanisms I
Many chemical reactions may look like simple straight-line equations, but have you ever wondered how the reactants are actually transformed into products at the molecular level? Imagine baking a meat pie – the final product is delicious, but the actual steps you follow (mixing, kneading, filling, folding and baking) are what lead to that result. Reaction mechanisms focus on those step-by-step transformations that molecules undergo during a chemical reaction.
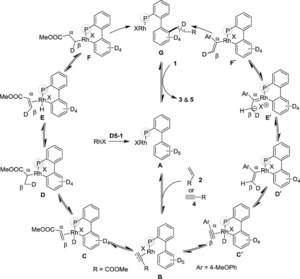
Meaning of Reaction Mechanisms
A reaction mechanism is a detailed description of the sequence of elementary steps that convert reactants into products. It explains how and why bonds are broken and formed during the course of a reaction, including the movement of electrons. Just like a football match is more than the final score — the goals, passes, and tackles matter — a chemical reaction is more than the final products, and the mechanism reveals what actually happens.
Types of Elementary Steps
Elementary steps are individual steps within a reaction mechanism. The most common steps include:
Nucleophilic Attack: When an electron-rich species (nucleophile) attacks an electron-poor species (electrophile). For example, imagine a thirsty person reaching for a cold sachet of pure water; the nucleophile is naturally attracted to the electrophile.
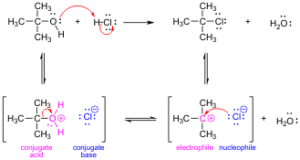
Bond Breaking (Heterolysis): When a bond breaks and both electrons go to one atom, producing ions. Think of friends sharing jollof rice and one person suddenly takes the entire plate, leaving the other with nothing – unequal sharing of electrons.
Bond Formation: The merging of two reactive species to form a stable bond.
Rearrangement: When atoms or groups within a molecule change positions to form a more stable structure.
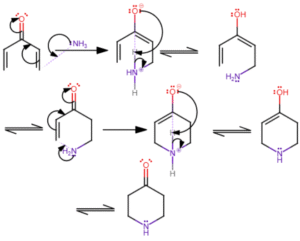
Reaction Intermediates
During mechanisms, temporary species called intermediates are formed. They include carbocations, carbanions, free radicals and carbenes. For instance, during alkene reactions, carbocations often appear briefly before forming the final product – like a temporary structure on a construction site before the building is completed.
Summary
- A reaction mechanism outlines the step-by-step sequence of events in a chemical reaction.
- Elementary steps include nucleophilic attack, bond breaking, bond formation and rearrangement.
- Intermediates (such as carbocations and free radicals) form temporarily during the reaction process.
- Understanding mechanisms helps predict product formation and reaction behaviour.
Evaluation
- What is a reaction mechanism?
- Mention two common elementary steps in a reaction mechanism.
- Define a reaction intermediate and give one example.
You’re doing excellently well. Keep up the great work – Afrilearn is cheering you on and can’t wait to continue with you in the next lesson!
