Back to: Organic Chemistry 200 Level
Welcome to class!
Hello and welcome back, brilliant one. It’s always a pleasure to learn with you. In our last class, we started talking about reaction mechanisms — like the hidden steps in a chemical drama. Today, we’re going further by focusing on some of the most common types of organic reaction mechanisms. You’ll see how different reactions take different paths and how we can follow each move like a detective solving a mystery. Let’s get right into it.
Reaction Mechanisms II
Nucleophilic Substitution Reactions (SN1 and SN2)
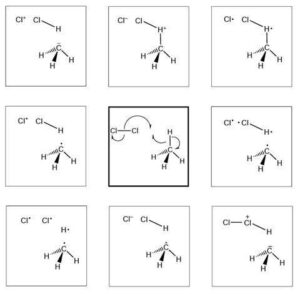
Let’s begin with something called nucleophilic substitution. Don’t let the big words scare you — just break it down.
Nucleophile: A species that loves positive centres and donates electrons.
Substitution: One group replaces another.
Think of a nucleophile as a helpful visitor trying to take the place of someone already living in a house. That’s exactly what happens in these reactions.
There are two main types:
SN1 (Substitution Nucleophilic Unimolecular)
Happens in two steps.
First, the leaving group goes away, forming a carbocation (positively charged intermediate).
Then the nucleophile attacks.
Common in tertiary halogenoalkanes.
Reaction rate depends only on the concentration of the substrate.
SN2 (Substitution Nucleophilic Bimolecular)
Happens in one step.
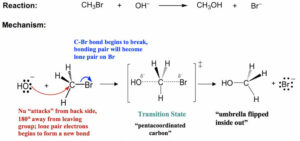
The nucleophile attacks at the same time as the leaving group leaves.
Common in primary halogenoalkanes.
The reaction rate depends on both the substrate and the nucleophile.
Real-life Example:
In drug design, changing a halogen on a carbon compound using nucleophilic substitution can turn a harmful compound into a useful medicine.
Electrophilic Addition Reactions
Next, we have electrophilic addition — mostly seen in compounds with double bonds, like alkenes.
Electrophile: A species that loves electrons and is positively charged or electron-deficient.
The double bond in alkenes acts like a magnet for electrophiles.
How it works:
The electrophile attacks the double bond.
A carbocation forms.
Another species (often a nucleophile) adds to the carbocation.
Example:
When bromine (Br₂) is added to ethene, the double bond breaks and each carbon gets a bromine atom. This type of reaction is used in testing for unsaturation using bromine water.
Free Radical Substitution
This happens mainly in alkanes, where hydrogen atoms are replaced by halogens in the presence of light or heat.
It occurs in three steps:
Initiation – Light breaks halogen molecules into radicals.
Propagation – The radical reacts with an alkane to produce new radicals.
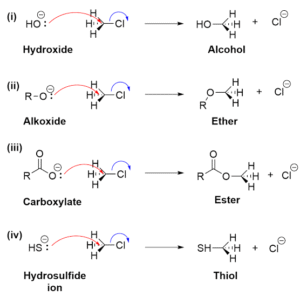
Termination – Two radicals combine to end the reaction.
Example:
Chlorination of methane in sunlight:
CH4+Cl2→UVCH3Cl+HCl\text{CH}_4 + \text{Cl}_2 \xrightarrow{\text{UV}} \text{CH}_3\text{Cl} + \text{HCl}
This method is used in industries to make products like chloroform and other solvents.
Summary
- SN1 and SN2 are types of nucleophilic substitution, depending on how the leaving group departs and the structure of the molecule.
- Electrophilic addition involves electron-deficient species reacting with alkenes, leading to the addition of atoms across the double bond.
- Free radical substitution uses radicals formed by UV light to substitute hydrogen atoms in alkanes.
- These mechanisms help explain how organic reactions occur in real life and in industrial applications.
Evaluation
- What is the difference between SN1 and SN2 reactions?
- Why are electrophilic addition reactions common with alkenes?
- List the three steps in a free radical substitution reaction.
- Give one example each of a nucleophile and an electrophile.
Well done, future chemist. You’ve just mastered the key reaction mechanisms that explain how so many everyday materials — from fuels to pharmaceuticals — are made. Keep asking questions, keep practising, and remember, with Afrilearn by your side, you’re never alone on this journey. See you in the next lesson!
