Back to: Organic Chemistry 200 Level
Welcome to class!
Hello, brilliant one! It’s so good to have you here again. You’ve been doing really well, and today’s lesson is another step forward in mastering Organic Chemistry. We’ve already learnt what alkenes and alkynes are — unsaturated hydrocarbons with double and triple bonds. Now, let’s look at how they react. These reactions are not just theory — they are the foundation of many things we use in our daily lives, from plastics to perfumes and even fuels. Let’s break it all down in a simple, clear way.
Reactions Of Alkenes & Alkynes
Why Are Alkenes and Alkynes Reactive?
The double bond in alkenes and the triple bond in alkynes are made up of one sigma (σ) bond and one or two pi (π) bonds. These pi bonds are weaker and easier to break, which makes alkenes and alkynes more reactive than alkanes.
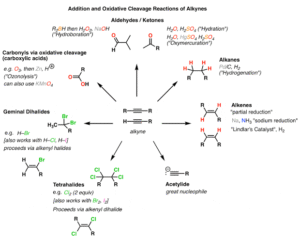
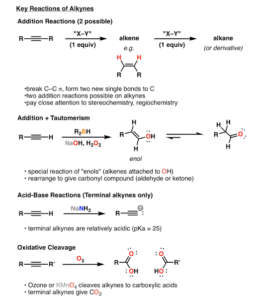
Because of this, they can undergo addition reactions — reactions where atoms or groups of atoms are added across the double or triple bond, turning them into saturated compounds.
Types of Reactions of Alkenes and Alkynes
Let’s go through the main reactions, using real-world-friendly examples.
1. Hydrogenation
This is the addition of hydrogen (H₂) to alkenes or alkynes in the presence of a metal catalyst like nickel or platinum.
It converts alkenes to alkanes.
Example:
Ethene + Hydrogen → Ethane
This process is used in making margarine by hydrogenating vegetable oils.
2. Halogenation
This involves adding halogens like chlorine (Cl₂) or bromine (Br₂) across the double or triple bond.
Test for Unsaturation:
When bromine water is added to an alkene or alkyne, the reddish-brown colour disappears — showing that a reaction has occurred. This is a common test to check for unsaturation.
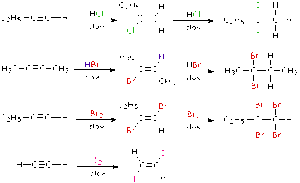
Example:
Ethene + Bromine → 1,2-Dibromoethane
3. Hydrohalogenation
This is the addition of hydrogen halides (like HCl, HBr) to alkenes or alkynes.
Example:
Ethene + HBr → Bromoethane
Note:
With unsymmetrical alkenes, the hydrogen attaches to the carbon with more hydrogen atoms already (this is called Markovnikov’s rule).
4. Hydration
Water (H₂O) is added in the presence of an acid catalyst, converting alkenes to alcohols.
Example:
Ethene + Water → Ethanol
This reaction is used industrially to make alcohol from ethene — a key step in producing alcoholic drinks, sanitisers and more.
5. Combustion
Alkenes and alkynes burn in oxygen to produce carbon dioxide and water, but because they have a higher carbon content than alkanes, they often burn with a sooty flame.
Example:
C₂H₂ + O₂ → CO₂ + H₂O
This is used in oxy-acetylene welding, where ethyne produces a very hot flame.
6. Polymerisation (For Alkenes)
Alkenes like ethene can undergo polymerisation — joining many small units to form a large molecule (polymer).
Example:
Ethene → Polyethene
Used to make plastic bags, containers, and bottles.
Summary
- Alkenes and alkynes are reactive due to their double and triple bonds.
- They undergo addition reactions such as hydrogenation, halogenation, hydrohalogenation, and hydration.
- They burn in air with a sooty flame because of high carbon content.
- Alkenes can undergo polymerisation to form useful materials like plastics.
- These reactions have real-world uses in food processing, medicine, fuel, and manufacturing.
Evaluation
- Why are alkenes and alkynes more reactive than alkanes?
- What is the result of adding bromine water to an alkene?
- State one real-life use of hydrogenation.
- Write a balanced equation for the combustion of ethyne.
Great job today! You’ve just learnt the powerful ways alkenes and alkynes react — and how those reactions affect the things we use every day, from cooking oils to plastic bottles. Keep learning with confidence, because every lesson brings you closer to your goals. Afrilearn is proud to walk this journey with you. See you in the next class!
