Back to: MICROBIOLOGY 200 LEVEL
Welcome to class!
Hello champion! I’m so proud of your consistency and curiosity. You’re doing something powerful—step by step, you’re unlocking the secrets of microbiology! Today’s topic is one of those quiet topics that work behind the scenes but are very important—Redox Reactions and Electron Carriers. If you’ve ever wondered how cells get energy from food, this is the magic behind it. Let’s go into it in a very simple and relatable way.
Redox Reactions And Electron Carriers
What Are Redox Reactions?
The word redox is a short form of reduction and oxidation, which are two processes that always happen together. Redox reactions are chemical reactions where electrons are transferred from one substance to another.
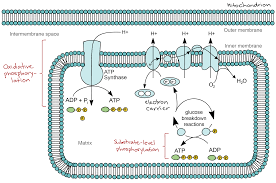
Let’s understand the two parts:
Oxidation is when a substance loses electrons.
Reduction is when a substance gains electrons.
You can remember it using a simple phrase:
OIL RIG – Oxidation Is Loss, Reduction Is Gain (of electrons).
Think of it like passing a hot meat pie from one person to another. The person who gives it away (oxidised) has less, while the one who receives it (reduced) now has more. The same thing happens in cells—but with electrons, not snacks!
Why Are Redox Reactions Important in Cells?
Cells need energy to survive, and that energy is stored in chemical bonds. When food like glucose is broken down during cellular respiration, electrons are released. These electrons pass through different molecules in the cell, and this transfer of electrons is what creates energy—in the form of ATP.
So, redox reactions help extract energy from nutrients. Without redox reactions, cells wouldn’t be able to generate ATP.
Who Are the Electron Carriers?
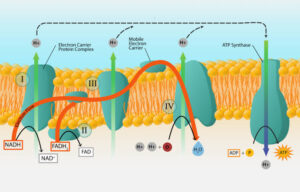
In your favourite Nollywood movie, there’s always that one character who carries gossip from one person to another. In cells, we have special molecules that carry electrons from one stage to another in energy production. These are called electron carriers.
The most important electron carriers in cells are:
NAD⁺ (Nicotinamide Adenine Dinucleotide)
– When it gains electrons, it becomes NADH (reduced form).
– It picks up electrons during reactions and carries them to where ATP is made.
FAD (Flavin Adenine Dinucleotide)
– When it gains electrons, it becomes FADH₂.
These carriers are like buses transporting passengers (electrons) to the power station (electron transport chain) to generate ATP.
Think of redox reactions like transferring airtime:
If you send ₦500 airtime to your friend, you’re oxidised (you’ve lost something).
Your friend is reduced (he has gained something).
The mobile network that moves the airtime is like the electron carrier—it makes sure the transfer happens smoothly.
Summary
- Redox reactions involve the transfer of electrons: oxidation is loss, reduction is gain.
- These reactions are key in producing energy from food.
- Electron carriers like NAD⁺ and FAD accept electrons and transport them during cellular respiration.
- The energy from redox reactions helps the cell make ATP, which powers all its activities.
Evaluation
- What is the full meaning of “redox”?
- What is the difference between oxidation and reduction?
- Name two major electron carriers and their reduced forms.
- Why are redox reactions important in cellular respiration?
You’ve done a fantastic job today! Learning about redox reactions may sound complex, but you’ve handled it like a true scientist. Keep that confidence burning bright—just like electrons powering a cell, your energy will carry you far. Afrilearn is proud to learn with you every step of the way. See you in the next class, star learner!
