Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s wonderful to see your enthusiasm shining again. Let me start with something familiar: imagine setting up chairs for a family gathering. The way the chairs are arranged depends on how many people are sitting and how much space they need. Similarly, atoms arrange themselves in specific shapes inside molecules, depending on how many electron pairs are around the central atom. Today, we’ll carefully study the shapes of important molecules and ions using the Valence Shell Electron Pair Repulsion (VSEPR) theory.
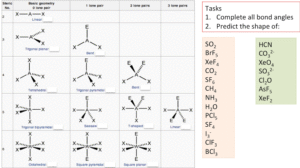
Shapes Of Molecules And Ions (E.G., CO₂, H₂O, NH₃, SF₆)
Carbon dioxide (CO₂)
Central atom: Carbon.
Electron pairs: 2 bonding pairs, no lone pairs.
The electron pairs arrange themselves on opposite sides of carbon.
Shape: Linear.
Bond angle: 180°.
Explanation: The two oxygen atoms pull equally on carbon, keeping the molecule straight.
Real-life connection: The gas you exhale in respiration is CO₂, essential in photosynthesis for plants.
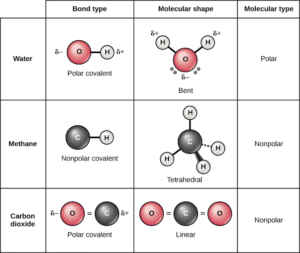
Water (H₂O)
Central atom: Oxygen.
Electron pairs: 2 bonding pairs + 2 lone pairs.
Lone pairs push harder, squeezing the bond angle smaller.
Shape: Bent (V-shaped).
Bond angle: ~104.5°.
Explanation: The lone pairs repel more strongly than bonding pairs, bending the molecule.
Real-life connection: Water’s bent shape makes it polar, explaining why it dissolves salts and sugar easily—earning its title as the universal solvent.
Ammonia (NH₃)
Central atom: Nitrogen.
Electron pairs: 3 bonding pairs + 1 lone pair.
Shape: Trigonal pyramidal.
Bond angle: ~107°.
Explanation: The lone pair pushes down the three hydrogens, tilting them into a pyramid shape.
Real-life connection: Ammonia is found in fertilisers used across Nigerian farms, boosting crop yield. Its shape affects how it interacts with other substances.
Sulphur hexafluoride (SF₆)
Central atom: Sulphur.
Electron pairs: 6 bonding pairs, no lone pairs.
Shape: Octahedral.
Bond angle: 90°.
Explanation: Six fluorine atoms sit symmetrically around sulphur like corners of an octahedron.
Real-life connection: SF₆ is used as an insulating gas in high-voltage electricity equipment because its symmetrical shape makes it extremely stable and non-reactive.
Summary
- CO₂: Linear, 180°.
- H₂O: Bent, ~104.5°.
- NH₃: Trigonal pyramidal, ~107°.
- SF₆: Octahedral, 90°.
- The shape depends on both bonding pairs and lone pairs, with lone pairs exerting stronger repulsion.
Evaluation
- State the shape and bond angle of CO₂.
- Why does water (H₂O) have a bent shape instead of linear?
- Name the shape of NH₃ and explain the role of the lone pair.
- How many bonding pairs surround the central atom in SF₆?
Well done today! You now understand why molecules are not flat symbols but 3D structures with unique shapes. With Afrilearn, you are not just memorising Chemistry—you are mastering it in ways that connect to real life. Keep your curiosity alive; the next lesson will make your knowledge even more powerful.
