Back to: Organic Chemistry 200 Level
Welcome to class!
Hi superstar! I’m truly glad you’re here today. Have you ever wondered how scientists can look at a substance and tell what it’s made of without even touching it? Sounds magical, right? Well, it’s not magic — it’s science. Today, we’re stepping into the fascinating world of Spectroscopy, a powerful tool chemists use to understand what’s inside a compound by simply studying how it interacts with light. You’re going to love how smart and simple this can be!
Spectroscopy Introduction
What Is Spectroscopy?
Spectroscopy is the study of how matter interacts with electromagnetic radiation — that’s just a fancy way of saying “how substances behave when exposed to light or energy”.
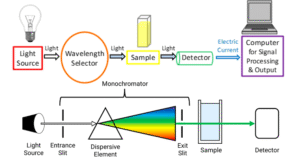
Every molecule has its own unique way of absorbing or reflecting light, just like every person has a unique fingerprint. By studying these light patterns, scientists can tell what atoms or bonds are present in a compound.
Think of it like this: Just as you can identify a friend by their voice on the phone, chemists identify molecules by the “signal” or “spectrum” they give off.
Why Is Spectroscopy Important?
Spectroscopy helps chemists to:
Identify unknown compounds.
Determine molecular structure.
Check the purity of a substance.
Monitor reactions in industries and research.
From drug development to forensic science, spectroscopy is used every day — even in hospitals to analyse blood samples.
Types of Spectroscopy
There are several types, depending on what part of light is used:
Ultraviolet-Visible (UV-Vis) Spectroscopy
Uses ultraviolet and visible light.
Tells us about conjugated systems (like double bonds in a row).
Useful in dyes, pigments, and detecting concentration of substances.
Infrared (IR) Spectroscopy
Uses infrared light.
Identifies functional groups in a molecule.
For example, a C=O bond or an O–H bond gives unique signals.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Uses radio waves in a magnetic field.
Gives detailed information about carbon and hydrogen environments in a molecule.
Very powerful in identifying organic compounds.
Mass Spectrometry (MS) – though not truly “spectroscopy”, it’s often grouped with it.
Tells the molecular weight and structure by breaking the molecule into pieces.
Real-life Connection
When pharmaceutical companies develop new drugs, they use spectroscopy to make sure they’ve made the right compound.
In food safety, spectroscopy checks if food contains harmful substances.
Even in space, scientists use spectroscopy to study stars and planets — from millions of kilometres away!
Summary
- Spectroscopy is the study of how substances interact with electromagnetic radiation (light and energy).
- It helps chemists understand structure, composition, and purity of compounds.
- Types include UV-Vis, IR, NMR, and Mass Spectrometry.
- It is widely used in medicine, industry, forensics, food testing, and even space research.
Evaluation
- What is spectroscopy in simple terms?
- Name two uses of spectroscopy in real life.
- Which type of spectroscopy is used to identify functional groups?
- Why is spectroscopy sometimes compared to a fingerprint?
Great work today, genius! You’ve just opened the door to one of Chemistry’s most exciting tools. With spectroscopy, you can see the invisible and understand the unknown — that’s the power of science. Keep learning with Afrilearn, and watch yourself grow into the scientist the world needs. You’ve got this — and we’re right here cheering you on. See you in the next class!
