Back to: Organic Chemistry 200 Level
Welcome to class!
Hello my dear friend, I’m really glad you’re here. Today’s topic is one that might sound like a big word at first, but trust me — it’s not as hard as it sounds. In fact, you’ll soon see how it connects to your everyday life in surprising ways. Whether it’s the medicine you take when you have a headache or the way your body processes food, today’s lesson helps us understand how shape and position matter in Chemistry. Let’s go on and make sense of it together.
Stereochemistry I
Introduction to Stereochemistry
Let’s start with this: Have you ever tried wearing your left shoe on your right foot? It doesn’t quite fit, does it? Even though both shoes look similar, they are not the same — that difference is because of how they’re shaped. This idea is what Stereochemistry is all about: the three-dimensional arrangement of atoms in molecules, and how this affects their properties and reactions.

In simple terms, Stereochemistry explains why some molecules behave differently even though they are made up of the same atoms. It’s the “chemistry of shape”.
Types of Isomerism
Now, before we dig deeper, let’s recall that isomers are compounds with the same molecular formula but different structures or arrangements. There are two major types:
Structural isomerism – where atoms are connected differently.
Stereoisomerism – where the connections are the same, but the spatial arrangement is different.
We’re focusing on stereoisomerism today.
Main Types of Stereoisomerism
There are two main types of stereoisomerism you should know:
Geometrical (cis-trans) isomerism
Optical isomerism
Let’s look at each briefly.
Geometrical Isomerism (Cis-Trans Isomerism)
This occurs in compounds with restricted rotation, especially around a double bond (like in alkenes). Imagine a molecule with two identical groups either on the same side (cis) or opposite sides (trans) of the double bond.
Example:
In but-2-ene:
Cis-but-2-ene has both methyl groups on the same side.
Trans-but-2-ene has them on opposite sides.
Even though both have the same atoms, they behave differently — for example, in their boiling points.
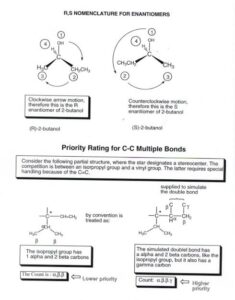
Optical Isomerism
Now, remember your left and right hands? They are mirror images but not superimposable. That’s how some molecules behave too — we call such molecules chiral.
Chiral molecules have:
A chiral centre (usually a carbon atom attached to four different groups)
Two mirror-image forms (called enantiomers) that cannot be placed on top of each other
These enantiomers rotate plane-polarised light differently — one to the left, the other to the right — which is why they are called optical isomers.
Why Does Stereochemistry Matter?
Stereochemistry is not just theory — it matters a lot in real life. For instance, one enantiomer of a drug might heal, while the other might cause harm. That’s why drug manufacturers must pay close attention to stereochemistry.
In food, scent, and even in the body’s natural chemistry, the arrangement of atoms makes all the difference.
Summary
- Stereochemistry is the study of how atoms are arranged in 3D space.
- Stereoisomerism occurs when molecules have the same connections but different spatial arrangements.
- Geometrical isomerism includes cis and trans forms, especially in alkenes.
- Optical isomerism involves chiral molecules with non-superimposable mirror images.
- Stereochemistry has real-world impact, especially in medicine and biology.
Evaluation
- What is stereochemistry, and why is it important?
- Differentiate between geometrical and optical isomerism.
- Give one real-life example where stereochemistry plays a role.
- What is a chiral centre?
Fantastic work today. You’ve taken a great step in understanding how molecules aren’t just about what they’re made of — but how they’re shaped and arranged. That’s powerful knowledge. Keep going strong, and remember, Afrilearn is here to help you succeed with every step. The next lesson will open even more doors of understanding. See you there!
