Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my brilliant learner! Today, we are starting an exciting journey into Stereochemistry. This branch of chemistry is all about the three-dimensional arrangement of atoms in molecules. Imagine if two people wore the same clothes but one wore them front-facing and the other inside out — the material is the same, but the arrangement is different. That is the essence of stereochemistry, and it can completely change how a molecule behaves in nature, in our bodies, and in chemical reactions.
Stereochemistry I
Meaning of Stereochemistry

Stereochemistry is the study of how atoms are arranged in space within molecules and how this arrangement affects their properties and reactions. It is sometimes called “3D chemistry” because it focuses on molecular shapes and orientations rather than just connectivity. Molecules with the same molecular formula and the same sequence of bonded atoms but different spatial arrangements are called stereoisomers.
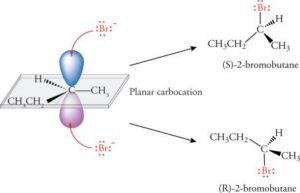
Types of Isomerism
Isomerism occurs when two or more compounds have the same molecular formula but different structures or arrangements. Structural isomerism is when the connectivity of atoms differs, while stereoisomerism occurs when the connectivity is the same but the arrangement in space differs.
Stereoisomerism Categories
Stereoisomers can be divided into two main types. Geometrical isomerism (also called cis–trans or E/Z isomerism) occurs due to restricted rotation around a double bond or in cyclic structures. In the cis form, similar groups are on the same side; in the trans form, they are on opposite sides. Optical isomerism occurs when molecules have non-superimposable mirror images, often because of a chiral centre — a carbon atom bonded to four different groups. These mirror-image isomers are called enantiomers.
Chirality
A molecule is chiral if it cannot be superimposed on its mirror image, just like your left and right hands. Chiral molecules rotate plane-polarised light in opposite directions: one enantiomer rotates it clockwise (dextrorotatory), the other anticlockwise (levorotatory). Chirality is extremely important in biology and medicine because enantiomers can have very different effects in the body.

Importance of Stereochemistry
In medicine, one enantiomer of a drug can be therapeutic while the other may be inactive or even harmful. In food science, stereochemistry determines taste and aroma. In materials science, it affects the strength, flexibility, and optical properties of polymers.
Summary
- Stereochemistry studies the 3D arrangement of atoms in molecules and its effects on properties.
- Stereoisomers have the same connectivity but different spatial arrangements.
- Geometrical isomerism arises from restricted rotation around double bonds or cyclic structures.
- Optical isomerism involves chiral molecules with non-superimposable mirror images.
- Chirality affects light rotation, biological activity, and chemical behaviour.
Evaluation
- Define stereochemistry in simple terms.
- Distinguish between structural and stereoisomerism.
- Explain the difference between cis and trans forms in geometrical isomerism.
- What is a chiral centre? Give an example.
- State one importance of stereochemistry in medicine.
You’ve taken your first confident step into the world of stereochemistry, and you’re already seeing how the smallest changes in arrangement can make a big difference. Afrilearn is proud of your progress, and you’re well on your way to mastering the fascinating 3D side of chemistry.
