Back to: Organic Chemistry 200 Level
Welcome to class!
Hello and welcome, my sharp scholar. It’s always a joy to have you here, ready to learn, grow and discover the secrets behind how our world works. In our last lesson, we looked at stereochemistry and saw how molecules that look alike can behave differently just because of their shape and arrangement. Today, we’re going a step further. We’ll look deeper into the types of optical isomers, how they affect light, and how we recognise and name these unique structures. Let’s begin.
Stereochemistry II
Understanding Optical Activity
Have you ever seen how a pair of sunglasses can block certain light but let some through? Something similar happens in Chemistry when certain molecules interact with plane-polarised light.
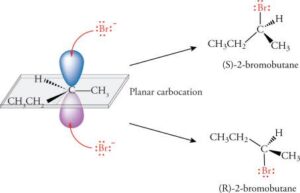
Molecules that can rotate plane-polarised light are said to be optically active. This means when light passes through them, they twist it either to the right (clockwise) or to the left (anticlockwise).
Here’s how we describe them:
Dextrorotatory (d or +): These rotate light to the right.
Laevorotatory (l or –): These rotate light to the left.
Even though the two molecules may contain exactly the same atoms and bonds, they behave differently when it comes to light. That’s the power of chirality.
Enantiomers vs Diastereomers
Let’s understand the types of stereoisomers under optical isomerism.
Enantiomers: These are mirror images of each other, like your left and right hands. They have the same physical properties (like boiling point, melting point), but they rotate light in opposite directions and may behave differently in biological systems.
For example, in some medicines, one enantiomer treats the illness, while the other could be inactive or even harmful. This is why identifying and separating them is so important in drug production.
Diastereomers: These are not mirror images of each other and are not superimposable. They often have different physical and chemical properties, unlike enantiomers. Not all chiral molecules have just one pair of mirror-image twins — sometimes there are more, and they don’t all behave the same.
Racemic Mixtures
A racemic mixture (or racemate) is a 50:50 mixture of two enantiomers — one dextrorotatory and one laevorotatory. Because the rotations cancel each other out, racemic mixtures are optically inactive overall.
Imagine pouring equal amounts of hot and cold water into a bowl. The final mixture might be warm, but it’s no longer hot or cold. That’s how racemic mixtures behave — the light rotation effect is neutralised.
Chirality in Nature and Industry
Stereochemistry plays a major role in:
Pharmaceuticals: Many drugs must be purified to include only the correct enantiomer.
Food and flavouring: The scent or taste of some compounds depends on their arrangement in space.
Biological systems: Our enzymes and DNA are chiral, meaning they interact differently with different isomers.
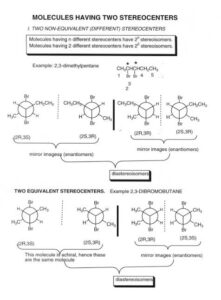
For example, the flavour of orange and lemon can come from molecules with the same formula but different spatial arrangement. Fascinating, right?
Summary
- Optical isomers rotate plane-polarised light either to the left or right.
- Enantiomers are mirror images that rotate light in opposite directions.
- Diastereomers are not mirror images and often have different properties.
- A racemic mixture contains equal amounts of enantiomers and is optically inactive.
- Stereochemistry matters in medicine, food, and living systems.
Evaluation
- What does it mean for a compound to be optically active?
- Explain the difference between enantiomers and diastereomers.
- Why are racemic mixtures optically inactive?
- Give two real-life areas where optical isomerism is important.
Excellent work! You’ve just uncovered the deep details of how molecular arrangement can change everything from taste to treatment. That’s how powerful Chemistry is — and you’re mastering it, one lesson at a time. Keep pushing forward, and remember, with Afrilearn, you have the tools to become unstoppable. I can’t wait to see you in our next lesson. Keep going!
