Back to: MICROBIOLOGY 200 LEVEL
Welcome to class!
Hello, superstar! It’s always a joy to have you here. You’re really growing into a brilliant microbiologist, and I’m super proud of how far you’ve come. Today, we’re going to learn about something special—Sulfur Reduction and Oxidation. Don’t worry about the big words; as always, we’ll break them down together in a fun and practical way that makes sense to you, just like gisting with a friend.
Sulfur Reduction And Oxidation
Why Is Sulfur Important?
Sulfur is one of the essential elements for life. It is a key part of some amino acids like cysteine and methionine, which help build proteins. It’s also found in important molecules like vitamins and coenzymes.
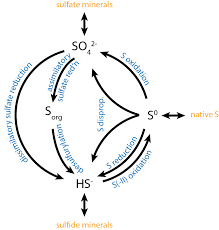
In the environment, sulfur goes through a natural recycling process—moving between living things, soil, water, and the air. This movement is known as the sulfur cycle, and two important parts of it are sulfur oxidation and sulfur reduction.
Sulfur Oxidation
Sulfur oxidation is when microorganisms convert reduced sulfur compounds (like hydrogen sulfide, H₂S) into oxidised forms like sulfate (SO₄²⁻). This process usually releases energy, which the microbes use to survive—especially those that live in places like hot springs, wetlands, or near volcanic areas.
Some of the bacteria involved include:
Thiobacillus
Beggiatoa
Acidithiobacillus
For example, Thiobacillus bacteria can take hydrogen sulfide (a gas that smells like rotten eggs) and convert it into sulfur or sulfate, which is less toxic and more usable by plants.
Think of this like frying smelly fish (H₂S) until it becomes dry, useful stockfish (sulfate). The transformation not only removes the bad smell but also provides something useful!
Sulfur Reduction
On the flip side, sulfur reduction is when bacteria do the opposite—they convert oxidised sulfur compounds (like sulfate) back into reduced forms like hydrogen sulfide. This process usually happens in anaerobic conditions—places where there is no oxygen, like deep soils, swamps, or inside the intestines of animals.
This type of respiration is used by sulfate-reducing bacteria like:
Desulfovibrio
Desulfotomaculum
These bacteria use sulfate instead of oxygen to “breathe”, and they release hydrogen sulfide as a by-product. That’s why you sometimes smell that rotten egg odour in gutters or stagnant water—it’s the work of sulfur-reducing microbes!
Why Does This Matter?
These processes help recycle sulfur in the environment.
They keep ecosystems healthy by breaking down waste and supporting plant nutrition.
In agriculture, sulfur-oxidising bacteria improve soil fertility.
In the oil industry, sulfur-reducing bacteria can cause problems like corrosion, but understanding them helps us find solutions.
Simple Nigerian Analogy
Imagine a mechanic workshop. One person is cleaning and restoring old parts (oxidation), while another is breaking parts down into raw materials (reduction). Both are helping to keep the whole system running smoothly—just like sulfur microbes do in nature!
Summary
- Sulfur oxidation is when microbes convert reduced sulfur (like H₂S) into sulfate, often releasing energy.
- Sulfur reduction is when microbes convert sulfate back into hydrogen sulfide, especially in oxygen-free environments.
- These processes are part of the sulfur cycle, helping to recycle nutrients and support life.
Evaluation
- What is sulfur oxidation?
- Name one microorganism involved in sulfur reduction.
- Where does sulfur reduction commonly occur?
- Why is the sulfur cycle important in nature?
Well done, champion! You’ve just learned how some of nature’s quietest workers—microbes—keep the sulfur cycle turning. That’s the mind of a true scientist! Keep going—you’re unstoppable. With Afrilearn, you’re learning the smart way, and your future is looking brighter every day. See you in the next class!
