Back to: MICROBIOLOGY 200 LEVEL
Welcome to class!
Hello my brilliant scientist-in-the-making! I’m so glad you’re here. You’ve been doing wonderfully, and today, we’re going to talk about something very essential—Thermodynamics and Microbial Energy Flow. Don’t worry, I’ll break it down simply for you. Just like how we need to understand how fuel powers a car, we need to understand how energy flows in microbes to see how they survive, grow, and function.
Thermodynamics And Microbial Energy Flow
What is Thermodynamics?
Thermodynamics is the study of energy and how it moves or changes form. In microbiology, we use thermodynamics to understand how microbes use energy for activities like growing, reproducing, and making new substances.
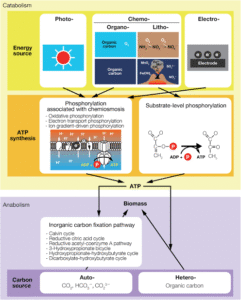
There are two main laws of thermodynamics that help us understand microbial energy flow:
1. First Law of Thermodynamics – Law of Energy Conservation
This law says energy cannot be created or destroyed, only changed from one form to another.
For example:
When a microbe breaks down glucose, the chemical energy stored in the glucose is not destroyed. It is converted into ATP, which the cell uses to do work.
Some of the energy is also released as heat, just like when your phone gets warm while charging.
2. Second Law of Thermodynamics – Law of Entropy
This law says that energy tends to become more disorganised over time. That is, every time energy is used, some of it is lost as heat, and things move towards disorder (entropy).
So, even though microbes can convert energy, they must do it efficiently so they don’t waste too much.
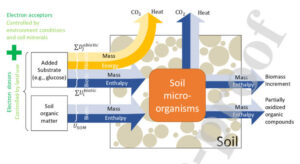
Microbial Energy Flow
Microbes get their energy in different ways, depending on what they eat and where they live. This flow of energy inside the microbe is what we call microbial energy flow.
Let’s look at how it happens step-by-step:
Energy Source
Microbes take in energy from light, chemicals, or organic matter (like sugars and fats).
Conversion
The energy is converted from one form to another—usually into ATP, the energy currency of the cell.
Usage
The ATP is then used to:
Build cell structures
Transport nutrients
Power movement
Reproduce
Energy Loss
Some energy is lost as heat during all these activities.
Imagine a young woman frying akara:
She uses kerosene (energy source).
The stove converts the energy into heat (conversion).
The heat cooks the akara (usage).
But some heat escapes into the air (loss).
Microbes work the same way. They must take in energy, convert it, use it, and deal with some waste along the way.
Free Energy (ΔG)
One more key term to know is Gibbs Free Energy (ΔG). It tells us whether a reaction will happen on its own or not:
If ΔG is negative, the reaction releases energy (exergonic) and can happen on its own.
If ΔG is positive, the reaction needs energy (endergonic) to happen.
Microbes prefer reactions with negative ΔG—they get energy from these and use it for survival.
Summary
- Thermodynamics explains how energy flows and changes form in microbes.
- The First Law says energy cannot be created or destroyed.
- The Second Law says some energy is always lost as heat.
- Microbial energy flow involves taking in energy, converting it to ATP, using it for life processes, and losing some as heat.
- Gibbs Free Energy helps us understand whether energy reactions in microbes will happen on their own.
Evaluation
- State the First and Second Laws of Thermodynamics in simple terms.
- What is microbial energy flow?
- What does it mean when ΔG is negative?
- Why do microbes need to convert energy into ATP?
You’ve done absolutely amazing today! Understanding how energy flows through microbes is a big step in mastering microbiology. Just like microbes manage their energy wisely to survive, you are wisely building your knowledge to thrive in life. Keep pushing forward—Afrilearn is right beside you on this beautiful learning journey. See you in the next class, superstar!
