Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m really glad you’re here today. Let me ask you something: have you ever stood at the beach in Lagos or any river bank and watched the waves rise and fall? Now imagine holding a football in your hand—you know it’s solid, it has weight, and you can throw it. But what if I told you that tiny particles like electrons behave like both the beach waves and the football? That strange but exciting idea is what scientists call wave-particle duality. Let’s break it down together.
Wave-particle Duality
What is Wave-Particle Duality?
Wave-particle duality is the concept that very tiny particles, like electrons and even light, can behave as both waves and particles depending on how we look at them. This is one of the most fascinating discoveries in modern physics and chemistry because it challenged the old idea that waves and particles were completely separate things.
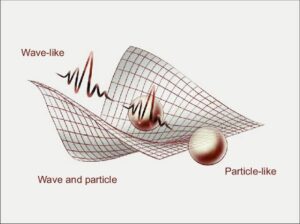
Light as a Wave and a Particle
For a long time, scientists debated about the nature of light. Isaac Newton believed light was made up of tiny particles. Later, others like James Maxwell showed that light also behaves like a wave, because it can reflect, refract, diffract, and even interfere with itself—just like water waves.
Then came Albert Einstein, who showed in the photoelectric effect that light can knock electrons out of metal surfaces as if it were made of particles called photons. Imagine sunlight on a solar panel: the energy of the photons makes electricity flow. That’s light acting like a particle.
Electrons as Waves and Particles
You may wonder: does this only apply to light? No! Electrons, which you know are particles inside atoms, can also behave like waves. In fact, Louis de Broglie suggested that if light (which was thought to be a wave) can behave like a particle, then matter (like electrons) can also behave like waves. This was confirmed in experiments where electrons were made to pass through two slits and they formed a wave-like interference pattern, just like light waves would.
Why is This Important?
Wave-particle duality is the foundation of quantum mechanics. It helps us understand how electrons are arranged in atoms, how chemical bonds form, and even how technologies like electron microscopes and semiconductors (used in your mobile phones) work.
Summary
- Wave-particle duality means tiny particles can behave like both waves and particles.
- Light shows wave properties (reflection, diffraction) and particle properties (photons in the photoelectric effect).
- Electrons also show wave properties, as proposed by de Broglie.
- This concept is central to quantum mechanics and modern technology.
Evaluation
- State two examples of light behaving as a wave.
- What experiment showed that electrons can behave like waves?
- Explain the meaning of a photon in simple terms.
Keep this in mind: what may look simple or ordinary at first—like light or an electron—can carry mysteries that power our entire modern world. You’re doing brilliantly by learning these concepts step by step. With Afrilearn by your side, you’re building the foundation for a future filled with discovery and innovation.
