CONTENT
- Nomenclature
- Preparation
- Properties
UNSATURATED HYDROCARBONS
These are hydrocarbons in which carbon atoms join with each other by multiple bonds. The multiple bonds can be double bonds e.g. Alkene or triple bonds e.g. Alkyne.
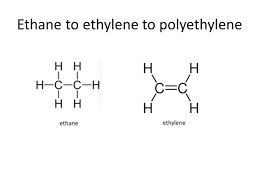
Alkenes e.g. Ethene
NOMENCLATURE
The process of naming in alkene is obtained by substitute “ane” in alkane with ‘ene’ e.g. Ethane changes to Ethene, propane to propene
PREPARATION (Lab. Preparation)
- Ethene is prepared by heating ethanol with excess concentrated tetraososulphate(VI)acid at 170o C. The acid acts as a dehydrating agent by removing water from the ethanol. Thus the process is called dehydration.
The reaction occurs in two stages
C2H5OH(aq) + H2SO4 (aq) C2H5HSO4 + H2O
C2H5HSO4 C2H4+ H2SO4.
The overall reaction is represented by the equation.
C2H5OH H2SO4 C2H4+ H2SO4
-H2O
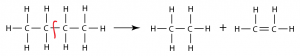
- Ethene can be prepared through cracking of Alkane e.g.
C3H8 C2H4 + CH4.
- By dehydration
e.g.
Physical Properties
- Ethene is a colourless gas with faint sweetish odour.
- It is sparingly soluble in water
- It is slightly less dense than air
- It has no action on litmus paper
Evaluation
- Write four (4) physical properties of Ethene
- How would you prepare a jar of ethane gas in the laboratory?
CHEMCIAL PROPERTIES
- Combustion
Ethene undergoes combustion/oxidation in air or in the presence of oxygen and produce carbon(iv)oxide and steam .
C2H4 + 3O2 2CO2 + 2H2O.
- Additional reaction
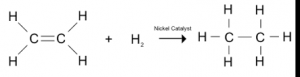
a. With hydrogen known as hydrogenation
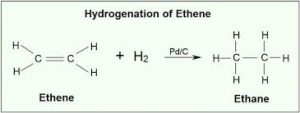
Ethene Ethane
b. With halogen know as halogenation
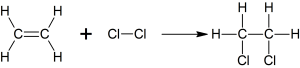
c. With Halides known as hydrogenation
e.g.
d. With acidified /Alkaline KMnO4 known as hydroxylation. It decolourses acidified KMnO4, but turns alkaline KMnO4 to green and result to ethane -1,2- diol.
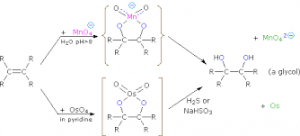
e. With Hydrogen peroxide in the presence of osmium trioxde to form ethan -1,2- diol.
f. With conc. H2SO4 known as hydration to produce fuming liquid of ethyl hydrogen sulphate.C2H4 + H2SO4 C2H5HSO4
When ethyl hydrogen sulphate is hydrolyzed, tetraoxosulphate (vi) acid and ethanol are produced.C2H5SO4 C2H5OH + H2SO4
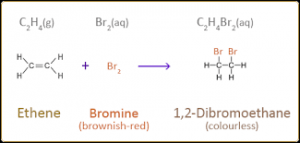
g. Ethene gas decolourizes bromine water to produce bromoethanol.
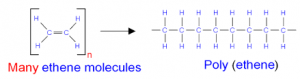
h. Polymerization of ethane to produce polythene.
i. With ozone known as ozomolysis to from ethane ozomides.
j. Ethene can also undergo additional reaction with oxygen in the presence of silver catalyst at about250oC to form epoxy ethane.
USES
- Used in the manufacture of plastics.
- Used in making synthetic rubber.
- Used to hasten the ripening of fruits.
- Used in the production of other organic compounds e.g. halo-alkane, ethane, ethanol.
Evaluation
- Describe the reaction of ethane with the following:
- Bromine water
- Chlorine water
- Acidified KMnO4
- State four (4) uses of Ethene.
READING ASSIGNMENT
New School Chemistry by O.Y. Ababio Pg 459-492
WEEKEND ASSIGNMENT
- The name of the organic compound with the structure below:
- Cis- but-2-ene
- Trans –cis-but-2-ene
- Trans-1-2- but-2-ene
- 1,2- dimethyl ethane.
- In the reaction given below:
C2H5OH Conc H2SO4 C2 H4 Conc H2SO4 is acting as
-H2O
- oxidizing agent B reducing agent C. Dehydrating agent D. Drying agent.
- One of the following is not a chemical property of ethane.
(a ) Polymerization (b) Substitutional (c ) Hydration (d) Addition.
- Function of the empty bottle during the preparation of ethane gas is
(a) to remove oxygen (b) to remove CO2 ( c) to prevent sucking back of the gas (d) None of the above.
- Additional reaction of hydrogen and ethene is known as
(a) polymerization (b) additional (c) combustion (d) hydrogenation
Theory
- Describe two (2) methods of obtaining ethene industrially.
2a. Write and name the geometric isomers of compound with the molecular formula C5H10
- With chemical equation only, show how ethane reacts with the following:
– ozone
– oxygen
– alkaline KMnO4
– Conc. H2SO4
