ALKANOIC ACIDS
CONTENT
Sources
Nomenclature
Structure
Preparation
Properties and Uses
ALKANOATES
General molecular formula, nomenclature, preparation, properties and uses.
SOURCES:
The alkanoic acid or carboxylic acids are also called fatty acids because some of them are found in natural fats and oils. They contain the functional group called carboxyl group.
NOMENCLATURE:
The IUPAC name of each homologue is obtained by changing the “-e” ending of the corresponding alkane to “-oic” acid e.g. mathanoic, ethanoic etc.
STRUCTURE:
Alkanoic acid has a general molecular fomular of CnH2n + 1COOH where n > 0. or RCOOH. Thus it has the following structure.
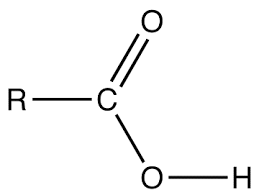
E.g. Ethanoic acid CH3COOH
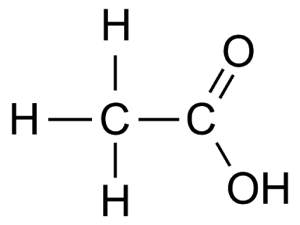
PREPARATION e.g. Ethanoic acid
Ethanoic acid can be prepared by the complete oxidation of ethanol by acidified sodium heptaoxo dichromate (VI) solution. The oxidation reaction is a two stages of reaction
- Ethanol oxidized to ethanol; CH3CH2OH O3 CH3CHO
- Ethanol oxidized to ethanoic acid; CH3CHO O3 CH3COOH
Or
CH3CH2OH + [O] CH3CHO + [O] CH3COOH
PHYSICAL PROPERTIES
- It is colourless liquid with a sharp and pungent smell.
- It has sour taste.
- It is soluble in water.
- It freezes into ice-like at temperature below 170C therefore called gluciaethanoic acid (anhydrous ethanoic acid).
- It has boiling point of 1180C
- It turns blue litmus papers to red.
CHEMICAL PROPERTIES
- It libratescarbon(IV) oxide from either trioxocarbonate (IV) or hydrogen trioxocarbonate (IV) salts. 2CH3COOH + Na2CO3 2CH3CONa + H2O + CO2.
- It librates hydrogen gas when it reacts with highly electropositive metals e.g.
Mg &Ca; 2CH3COOH + Ca (CH3COO)2Ca + H2.
- As an acid, it neutralizes boxes or alkalis to form salts known as ethanoate and water only
CH3COOH + NaOH CH3COONa + H2O.
- It reacts with alkanols to form ester e.g.
- CH3COOH + CH3CH2OH CH3COOCH2CH3 + H2O
- Reduction:
It can be reduced to ethanol in the presence of lithium tetrahydrido aluminate III as reducing agent (LiAlH4)
CH3COOH + 4H CH3CH2OH + H2O
- It reacts with chlorine successively to form chloroethanoic acid e.g.
CH3COOH + Cl2 CH2ClCOOH + HCl
CH2ClCOOH + Cl2 CHCl2 COOH + HCl
CHCl2 COOH + Cl2 CCl3COOH + HCl
EVALUATION
- (a) State four (4) chemical properties of ethanoic acid.
(b) Give two (2) physical properties of ethanoic acid.
- How would you prepare ethanoic acid in the laboratory.
CLASSIFICATION OF ALKANOIC ACID
Alkanoic acids are classified based on the number of carbonxylic groups present per molecules.
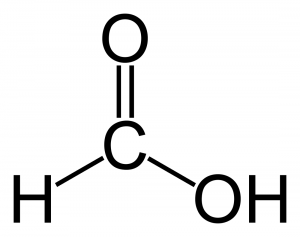
- Monocarboxylic Acids: these have one carboxylic group per molecule e.g. methanoic acid (HCOOH)
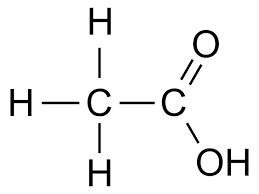
Ethanoic acid CH3 COOH
2. Dicarboxylic Acids: these have two carboxyl groups per molecules e.g. ethan -1, 2-dioe acid
(oxalic acid)
COOH
or
COOH
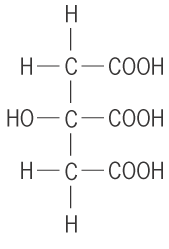
- Tricarboxylic acids: these have 3 carboxylic acid per molecule e.g. 2-hydroxy propan 1,2, 3-tricaboxylic acid.
N.B: Two important aromatic carboxylic acid are
(1) Benzoic acid
(2) 2-hydroxy benzoic acid
USES OF ETHANOIC ACID
- It is used in making compounds like cellulose ethanoate, dyes etc.
- It is used as organic solvent.
- It is used in the food industries for preserving and flavoring food.
- Used for coagulating rubber latex.
EVALUATION
- Give three (3) classes of alkanoic acid.
- State four (4) uses of ethanoic acid.
READING ASSIGNMENT
New School Chemistry by O.Y. pages.504-506
ALKANOATES
General molecular formula.
The alkanoates are called esters. They have general molecular formula of RCOOR’.
It has structural formula of
e.g. ethyl ethanoate CH3COOCH2CH3
NOMENCLATURE
Naming of alkanoates are obtained by substituting “e” ending in alkane with “oates” in alkanoates.
Preparation e.g. ethyl ethanoate.
Ethyl ethanoate is prepared by the esterification between ethanol and glaciusethanoic acid at 1500C in the presence of concentrated tetraoxosulphate (VI) acid
C2H5OH + CH3COOH CH3COOC2H5 + H2O
PHYSICAL PROPERTIES
- Ethyl ethanoate is a colourles volatile liquid with a pleasant smell.
- It is slightly soluble in water.
- It has boiling point of 750C.
CHEMICAL PROPERTIES
- Hydrolysis.
Ethyl ethanoate can be hydrolysed by water to produce ethanoic acid and ethanol.
CH3COOC2H5 + H2O CH3COOH + C2H5OH.
N.B: If an alkali is used instead of water, it will produce the salt of the acid e.g.
CH3COOC2H5 + NaOH CH3COONa + C2H5OH
- REACTION WITH AMMONIA
Ethyl ethanoate reacts with ammonia to produce ethanol and then amide
CH3COOC2H3 + NH3 C2H5OH + CH3COOH2
- REDUCTION
Ethyl ethanoate can be reduced by hydrogen from lithium tetrahydridoalluminute (III) as reducing agent
CH3COOC2H5 + 4[H] 2C2H5OH
USES OF ALKANOATES/ESTERS
– They are used as food flavours.
– Used in perfumes and cosmetics
– Used as solvent for cellulose nitrate.
– Used for quick-drying substances like paints, nail varnishes etc.
EVALUATION
- Write the general structure of the ester.
- Write a balanced equation for the reaction between propanol and butanoic acid.
(a) Name the products formed.
(b) What type of reaction is involved?
READING ASSIGNMENT
New School Chemistry by Osei Yaw Ababio page.504-509
WEEKEND ASSIGNMENT
- The name of (CH3)2 CHCOOH is
- Propane acid B. 2-methylhutanoic acid C. Dimethyl butanoic acid
- Propanoic acid
- Citric acid appears in unripe orange while enthanoic acid appears in
- Unripe pawpaw B. Carrot C. Vinegar D. Rice
- Esters are employed in the following except.
- Making perfumes B. Making cement C. Nail varnishes D. Making yeast
- An alkanoic acid reacts reversibly with an alkanol to produce.
- a salt B. an ester C. a sugar D. an alkene
- Ethan-1, 2-dioe acid is
- a mineral acid B. dicarboxylic acid C. citric acid D. a soap
THEORY
1a. Give the formula of ethanoic acid and indicate its functional group.
- Ethanoic acid reacts with both sodium hydroxide and ethanol, suing equations to comparethe reactions and classify the products.
2a. Ethylethanoate reacts with both water and alkali; using equation to compare the reaction.
- What happens when ethanoic acid is heated strongly with soda-line.

This was very helpful
Thanks a lot