Back to: PHYSICS SS1
Welcome to class!
In today’s class, we will be talking about density and relative density. Enjoy the class!
Density and Relative Density

What is density?
Density is by definition the ratio of the mass and volume of a body. SI dimension of density is kilogram per cubic meter – kg / m3. Density is a constant characteristic of a body, but since it depends on the temperature, density is commonly declared together with the temperature at which it is determined. The density of a substance is influenced by composition, temperature, physical state, allotropic shape, electric field, etc. Density is sometimes regarded as specific weight, with a measurement unit of Newton per cubic meter.
The concept of density
The figure below shows two identical flasks one filled with water to 250cm3 mark and the other filled with kerosene to the same 250cm3 mark when measured in electronic balance the flask filled with water is found to be heavier than that filled with kerosene why? The answer is in finding the mass per unit volume of kerosene and water in respective flasks.
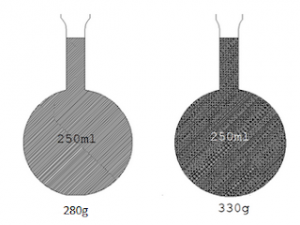
The mass of the flask filled with water is 330g, and the mass of flask filled with kerosene 280g, the empty flasks were measured and found to be 80g, therefore, the mass of water only is 250g and kerosene only is 200g.
Mass per unit volume of water is 250g/ 250cm3 this is 1g/cm3.
Mass per unit volume of kerosene is 200g/ 250cm3this is 0.8g/cm3.
The results 1g/cm3 and 0.8g/cm3 are the densities of water and kerosene respectively.
Therefore the density of a substance is the mass per unit volume of a given substance.
The SI unit of density is kilogram per meter cubic (kg/m3) also gram per centimetre cubic (g/cm3). The symbol for density is rho (ρ) ρ=mass/volume.
Worked examples
(1) A block of ice with volume 5.5m3 has a mass of 5060kg find the density of ice.
Solution
Volume of block=5.5m3
Mass of block=5060kg
Density=mass /volume
=5060/5.5m3.
=920kg/m3.
The density of ice is 920kg/m3.
(2) A silver cylindrical rod has a length of 0.5m and radius of 0.4m. Find the density of the rod if its mass is 2640kg.
Solution
Mass of cylinder=2640kg
The volume of cylinder= πr²h
=3.14 x 0.42 x 0.5
=0.2512m3
Density=mass/volume
=10509 kg/m3
(3) A stone has a mass of 112.5g.when the stone totally immersed in water contained in measuring cylinder displaced water from 50cm3 to 95cm3.find the density of the stone.
Solution
Mass of the stone=112.5g
Volume of stone=95cm3-50cm3=45cm3
Density=mass/volume =2.5g/cm3.
Density bottle
The density bottle (pycnometer) consists of ground glass stopper with a fine hole through it.
The function of the fine hole in a stopper is that, when the bottle is filled and the stopper is inserted, the excess liquid rises through the hole and runs down outside the bottle, by this way the bottle will always contain the same volume of whatever the liquid is filled in providing the temperature remains constant.
The bottle is used to measure density and relative density, relative density is the comparison of one density to another, thus a density of a given volume of a substance to a density of the equal volume of the referenced substance, for example, a ratio of a density of a given volume of a substance to a density of an equal volume of water, this is referred to a relative density of a given substance or Specific gravity of a given substance. The term specific gravity is used when the reference substance is water.
Determination of densities of granules and sand
To find the density of sand or granules such as lead shots a density bottle is used as follows
- Find the mass of empty dry density bottle mo
- Put some granules and find the mass m1 =( mass of empty bottle + mass of granules)
- Pour water in the bottle until it is full and find mass m2 = (mass of bottle + mass of granules + mass of water on top of granules)
- Find the mass of bottle filled with water only m3 =( mass of bottle + mass of water)
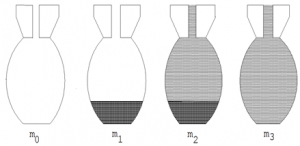
The mass of sand = (m1 – m0)g
Mass of water above the sand = (m2 – m1) g
Mass of water filling the bottle = (m3 – m0) g
Since the density of water is 1g/cm³
Volume of sand = [(m1+m3)-(mo+m2)]/1g/cm³
= [(m1+m3)-(mo+m2)] cm³
Density=mass/volume
Relative density
What is relative density?
Relative density is the ratio between the density of a measuring substance and the density of some other reference substance at a given temperature (typically water). It refers to the ratio of the density of the substance to the density of water.
Relative density =
d = density of the substance
dw = density of water
the relative density of water = 1.0
All metals and most solids have R.D greater than 1 while air and several liquids have R.D. less than 1.
Units: R.D. bears no Units
Other definition of relative density
The relative density of a substance is the ratio of the density of a substance to the density of water.
Or
The relative density of a substance is the ratio of the mass of any volume of a substance to the mass of an equal volume of water.
To measure the relative density of liquid by density bottle
- Find the mass of empty bottle = m0
- Find the mass of bottle and liquid = m1
- Empty the bottle and rinse it with water
- Fill the bottle with water and find mass m2
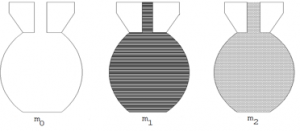
Mass of liquid= (m1 – m0)g
Mass of equal volume of water= (m2 – m0)g
Since comparison of density is done with water (referenced substance) the other name of the ratio is the specific gravity of a given substance. Because the density of water is 1g/cm³.Relative density has no units it is simply a number or ratio.
In our next class, we will be talking about Upthrust and Archimede’s Principle. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.

a word meaning of density
what about mass
i really appreciate this class
is the measurement of the amount of a substance per unit volume