Back to: CHEMISTRY SS1
Welcome to class!
In today’s class, we will be talking about the introduction to the mole concept. Enjoy the class!
Introduction to the Mole Concept
The mole
A mole is a number of particles of a substance which may be atoms, ions, molecules or electrons. This number of particles is approximately 6.02 x 1023 in magnitude and is known as Avogadro’s number of particles.
The mole is defined as the amount of a substance which contains as many elementary units as there are atoms in 12g of Carbon-12.
Relative atomic mass
The relative atomic mass of an element is the number of time the average mass of one atom of that element is heavier than one-twelfth the mass of one atom of Carbon-12. It indicates the mass of an atom of an element. E.g., the relative atomic mass of hydrogen, oxygen, carbon, sodium and calcium are 1, 16, 12, 23, and 40 respectively.
The atomic mass of an element contains the same number of atoms which is 6.02 x 1023atoms; 1 mole of hydrogen having an atomic mass of 2.0g contains 6.02 x 1023 atoms.
Self-evaluation:
- Define the relative atomic mass of an element
- State the relative atomic mass of the following elements: potassium, chlorine, silver, lead, phosphorus and nitrogen
Relative molecular mass
The relative molecular mass of an element or compound is the number of times the average mass of one molecule of it is heavier than one-twelfth the mass of one atom of Carbon-12
It is the sum of the relative atomic masses of all atoms in one molecule of that substance. It is also called the formula mass. The formula mass refers not only to the relative mass of a molecule but also that of an ion or radical.
Calculation
Calculate the relative molecular mass of:
- Magnesium chloride
- Sodium hydroxide
- Calcium trioxocarbonate
[Mg=24, Cl=35.5, Na=23, O=16, H=1, Ca=40, C=12]
Solution:
- MgCl2 = 24 + 35.5×2 = 24 + 71 = 95gmol-1
- NaOH = 23 + 16 + 1 = 40gmol-1
- CaCO3 = 40 + 12 +16×3 = 100gmol-1
Self-evaluation:
- What is the relative molecular mass of a compound?
- Calculate the relative molecular mass of (a) NaNO3 (b) CuSO4.5H2O
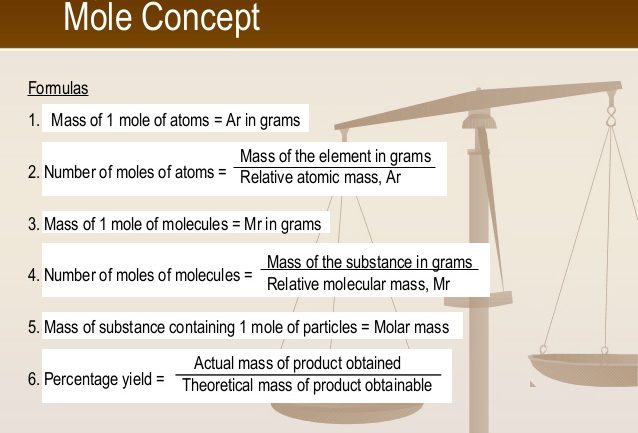
Molar volume of gases
The volume occupied by 1 mole of a gas at standard conditions of temperature and pressure (s.t.p.) is 22.4 dm3. Thus 1 mole of oxygen gas of molar mass 32.0gmol-1 occupies a volume of 22.4dm3 at s.t.p. and 1 mole of helium gas of molar mass 4.0gmol-1 occupies a volume of 22.4 dm3 at s.t.p.
Note: When the conditions of temperature and pressure are altered, the molar volume will also change. Also, standard temperature = 273K and standard pressure = 760mmHg.
Relationship between quantities
- What is the number of oxygen atoms in 32g of the gas (O=16, NA = 6.02 x 1023)?
Solution:
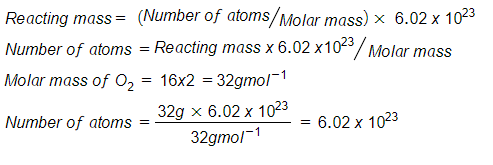
The number of oxygen atoms is 6.02 x 1023
Self-evaluation:
- Define the molar volume of a gas
- How many molecules are contained in 1.12dm3 of hydrogen gas at s.t.p?
Percentage of an element in a compound
The percentage composition of an atom in a compound is the amount of the atom expressed in percentage.
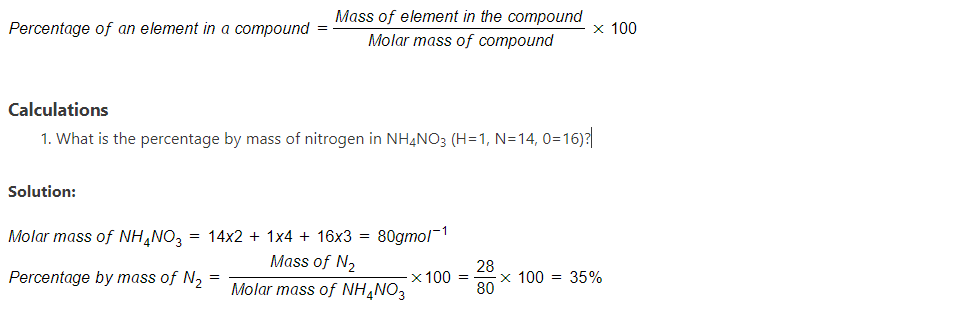
- Calculate the percentage by mass of water of crystallization in MgSO4.7H2O (Mg=24, S=32, 0=16, H=1)
Solution:
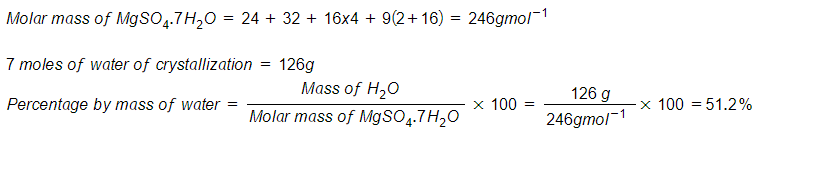
General self-evaluation
- What is the number of molecules in 6.4g of SO2 (NA = 6.02 X 1023)?
- What is the volume in cm3 of 2.2g of CO2 at s.t.p. (C=12, O=16)?
- Determine the percentage by mass of oxygen in Al2(SO4).2H2( Al=27, S=32, O=16, H=1)
Reading assignment
New School Chemistry for Senior Secondary Schools by O. Y Ababio, Pg 28-31
Weekend assignment
- What is the relative atomic mass of potassium A. 40 B. 39 C. 32 D. 24
- An element with relative atomic mass 108 is A. Ca Cl B. Na C. Ag D. Al
- Modern standard element with which chemist define relative atomic mass is A.12C B.C133H D.16O
- Calculate the relative molecular mass of CH3A. 60gmol-1B. 70gmol-1C. 80gmol-1D. 90gmol-1
- How many moles are there in 12g of CO2 (C=12, 0=16)?A. 0.27 B. 0.47 C. 0.16 D. 0.32
Theory
- Calculate the actual number of atoms contained in 2.8dm3 of chlorine (Molar volume of gas = 22.4dm3, NA = 6.02 X 1023)
In our next class, we will be talking about Writing And Balancing Chemical Equations. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.

The class lesson was interesting and enthusiastic.It follows a step by step instructions and solutions.
I wish the answers to the class work re given
I LOVED THE CLASS BUT THIER IS A PART IN MOLE CONCEPT YOU DID NOT EXPLAIN OR SOLVE ON I ONLY SAW QUESTIONS ON THEM BUT NO WORRIES THANKS ONCE MORE I ENJOYED THE CLASS
I AM SO MOUCH HAPPY
Was nice
I did like the class but no answer to the classwork
can you later give answers to the questions so that we can know where we are getting it wrong
I need further explanation about the questions