Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about Electrochemical Cells. We hope you enjoy the class!
CONTENT
- Electrolytic and Electrochemical Cells with their differences.
- Drawing and Writing of Cell Diagrams.
- Standard Electrode Potential.
- Calculation of e.m.f of a Cell.
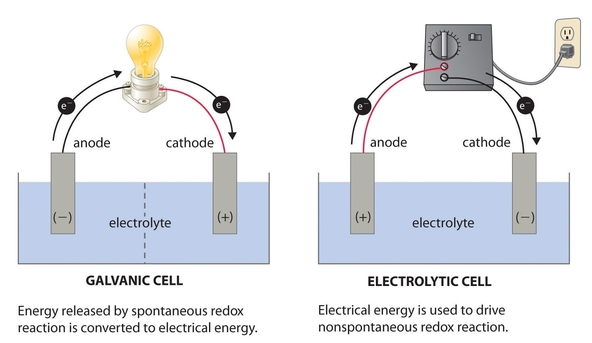
ELECTROLYTIC AND ELECTROCHEMICAL CELL
An electrolytic cell is a device in which electrical energy is converted to chemical energy. An electrochemical cell is a device in which chemical energy is converted to electrical energy.
DIFFERENCES BETWEEN ELECTROLYTIC CELL AND ELECTROCHEMICAL CELL
| ELECTROLYTIC CELL | ELECTROCHEMICAL CELL | |
| 1. | Electrical energy is converted to Chemical energy is converted energy chemical energy. | Chemical energy is converted to electrical energy. |
| 2. | The cathode is negative electrode while the anode electrode is the positive electrode. | The cathode is positive while the anode is the negative electrode. |
| 3. | No salt bridge is required | Salt Bridge or porous partition required. |
| 4. | The electrolyte may not contain ions of the metal electrode used | The electrolyte must contain metal ions of metal electrode used. |
| 5. | Electrons are pushed by an external source Electrons are generated by at the anode. | Electrons are generated by oxidation such as a battery. |
Note: An electrolytic cell is also known as a galvanic cell it consists of two half cells
- an oxidation half cell
- a complementary reduction half-cell.
DRAWING AND WRITING OF CELL DIAGRAMS
A typical electrochemical cell consists of two electrodes (cathode and anode) and their electrolytes. The electrodes are connected by means of a salt bridge. A typical electrode or half-cell is a device in which an element (metal) is in contact with its own ions. A typical metallic electrode is symbolically represented as:
M(s)/Mn+ (aq) or Mn+ (aq)/M(s)for metals dipped into solutions of their own ions.
Conventional notation is used to describe an electrochemical cell without drawing. Example:
X/Xn+ //Yn+/Y
The single vertical lines (/) represents metal and metal ion interphase while the double lines (//) represents the salt bridge or a porous partition.
For a simple electrochemical cell, there is a flow of electrons from the anode (negative electrode) to the cathode (positive electrode). Also, for an electrochemical to produce electricity, the anode element must be more reactive than the cathode element. That is, the anode element must be higher in the electrochemical series than the cathode.
For a given net reaction as shown below
Zn(s) + Cu 2+(aq) Zn2+(aq) + Cu(s)
The Cell notation for the system is
Zn/Zn2+ // Cu2+/ Cu
Since Zn is higher up in the electrochemical series, it will serve as the anode while copper will serve as the cathode. The anodic half-cell is always written on the left-hand side while the cathodic half-cell is always the right-hand side.
The diagram below shows the drawing of the above electrochemical cell:
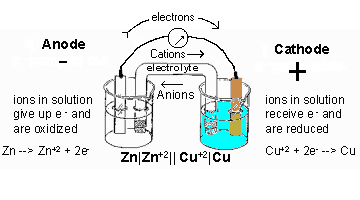
Salt Bridge
In an electrochemical cell, instead of using the porous partition to maintain the electrical neutrality of the half-cell electrolytes, the half-cells can be linked by a SALT BRIDGE. Salt bridge is a filter paper soaked in sodium chloride solution or KCl or NH4NO3.
Functions of a salt bridge
- It helps in completion of electric circuit
- It enables the migration of ions from one-half cell to the other in order to maintain electrical neutrality in the solution when current flows in the setup
EVALUATION
- State two differences between electrolytic and electrochemical cells.
2. Write a cell notation for the equation below
Zn(s) + 2Ag+(aq) Zn2+(aq) + Ag(s)
3. Draw the cell diagram for the cell above.
ELECTRODE POTENTIAL
This is the potential difference that is set up between a metallic electrode and its electrolyte. Example
- Copper ion/copper system:
If a copper plate is dipped into a CuSO4 solution, the net reaction is
Cu2+(aq) + 2e– Cu(s)
That is: Cu2+(aq)/Cu(s)
2. Zinc ion/zinc system:
If a zinc plate is dipped into a ZnSO4 solution; the net reaction is
Zn2+(aq) + 2e– Zn(s)
That is: Zn2+(aq)/Zn(s)
Standard Electrode Potential
The electrode potential of a given system depends on
- the overall energy change
- the concentration of ions in the solution
- the temperature
Standard electrode potential (E0) is used to compare the electrode potential values of different metal ions/metal system.
In measuring standard electrode potential of metal ion/metal system difference (in volts) which exists between the metallic electrode and the standard hydrogen electrode, the standard hydrogen electrode potential is considered to have an arbitrary value of zero.
The standard electrode potential of a metal ions/metal system is the potential difference set up between the metal and its ion compared to the electrode potential of H+(aq)and H2 gas system.
Metal ion/Metal E0
K+(aq)/K(s) -2.92V
Ca2+(aq)/Ca(s) -2.87V
Na+(aq)/Na(s) -2.71V
Zn2+(aq)/Zn(s) -0.76V
2H+(aq)/H2(g) 0.00
Cu2+(aq)/Cu(s) +0.36V
Ag+(aq)/Ag(s) +0.80V
The electrode potential of Cu = +0.34V as electron flows from the hydrogen electrode to copper electrode. At platinum hydrogen electrode
H2(g) 2H+ + 2e–(oxidation)
At the copper electrode
Cu2+(aq) + 2e– Cu(s) (reduction)
Overall reaction: Cu2+(aq)+ H2(g) Cu(s) + 2H+(aq)
NOTE: Eǿtotal is negative if electron flows from the metal electrode and positive when it flows from hydrogen electrode to the metal electrode
EVALUATION
- Define electrode potential
- List two functions of a salt bridge.
E.M.F OF A CELL
When two half-cells are joined together through a salt-bridge, the e.m.f. of the cell thus formed is the algebraic difference between the two electrode potentials.
The e.m.f. of the cell formed by the system Zn(s)/Zn2+(aq) & Cu2+(aq)/Cu(s) is defined as the standard electrode potential of the right-hand electrode minus the standard electrode potential of the left-hand electrode.
Eǿtotal = EǿR– EǿL
= +0.34 – (-0.76)
=+1.10V
A positive e.m.f. indicates that the left- hand electrode (zinc) is partially capable of reducing copper (ii) ions to copper
But for the system Cu(s)/Cu2+(aq)/&/Zn2+(aq)/Zn(s)
Eǿtotal = EǿR – EǿL= -0.76-0.34= -1.10V
A negative e.m.f. indicates that the left-hand electrode (copper) cannot reduce zinc ions to zinc atom.
In general, when this convention is used, then a positive e.m.f indicates that the reaction is thermodynamically feasible as written down from left to right.
A negative e.m.f implies that the reaction is thermodynamically impossible as written down.
GENERAL EVALUATION/REVISION
- Calculate the e.m.f of a cell when zinc half cell is connected with a salt bridge to a copper half-cell if the Eө of Zn2+(aq)/Zn(s) = -0.76V and Ag+(aq)/Ag(s) = +0.80V
- Give two examples of primary cells
- Define electrode potential and list two functions of a salt bridge.
- What is standard electrode potential?
- State Aufbau principle and Hund’s rule of maximum multiplicity.
READING ASSIGNMENT
New School Chemistry for Senior Secondary Schools by O. Y. Ababio, pages 215-216
WEEKEND ASSIGNMENT
SECTION A: Write the correct option ONLY
- A Porous material used to maintain electrical neutrality in a solution of an electrochemical cell is called A. Pot B. Salt bridge C. Paper D. none of the above
- The element with the electronic configuration 1s22s22p63s23p64s2 is a/an A. group two element B. transition metal C. alkaline earth metal D. none of the above
- Oxidation takes place at the anode during electrolysis because anode A. is deficient in electrons B. is different in protons C. has excess electrons D. none of the above
- The potential difference set up between metallic electrode and the electrolyte solution is known as A. Lead accumulator B. electrode potential C. Voltammeter D. none of the above
- In an electrochemical cell, the cathode is made A. negative B. positive c. neutral D. none of the above
SECTION B
- State THREE differences between an electrolytic cell and an electrochemical cell
- Mention TWO function of a salt bridge
- What is the voltage of the cell represented as Ca(s)/Ca2+(aq)//Cu2+(aq)/Cu(s) given that electrode potential of Ca2+(aq)/Ca(s) is -2.87V and that of Cu2+(aq)/Cu(s) is +0.36V
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about the Application of Electrochemical Cells. We are very much eager to meet you there.

I have learnt a lot , thank you so much.