Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about Electrolysis. We hope you enjoy the class!
CONTENT
- Meaning of Electrolysis
- Definition of Terms
- Preferential Discharge of Ions during Electrolysis.
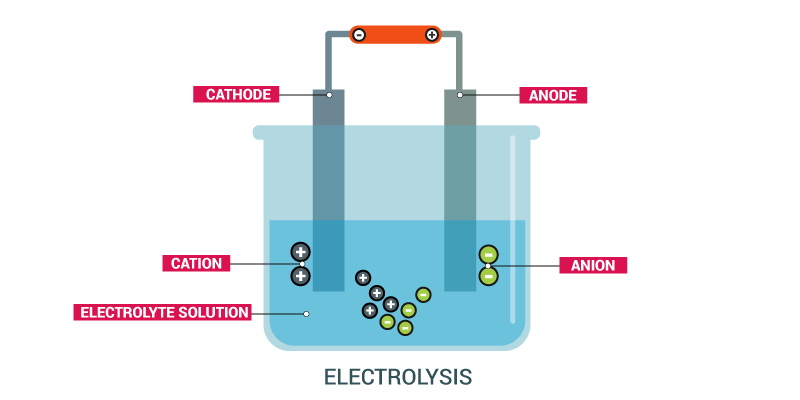
ELECTROLYSIS
Chemical decomposition of a substance can be brought about by heating. Similarly, an electrical effect can also be used to bring about chemical decomposition of substances. The Effect of electricity on matter is studied under electrolysis.
DEFINITION OF TERMS:
- ELECTROLYSIS: is defined as the chemical decomposition of a compound (electrolyte) brought about by the passage of direct current through either a solution or the molten form of the compound.
- ELECTROLYTE: An electrolyte is a compound which conducts electricity and is decomposed in the process. To behave as an electrolyte, the compound must be in a liquid form either as a molten compound or an aqueous solution of the compound. A non-electrolyte does not conduct electricity in this manner.
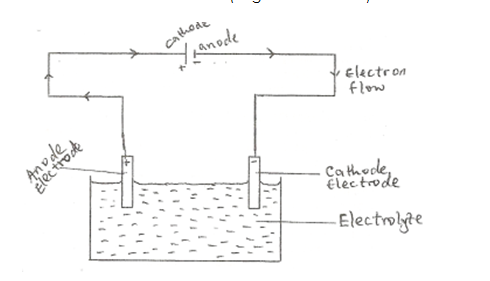
- ELECTRODES: Electrodes are conductors in the form of wires, rod or plates through which an electric current leaves or enters the electrolyte.
- ANODE: Anode is the positive electrode by which the electrons leave an electrolyte (or by which conventional current enters the electrolyte). It is the electrode which is joined to the positive terminal of the direct current supply.
- CATHODE: This is the negative electrode by which electrons enters the electrolyte. It is the electrode which is joined to the negative terminal of the electric supply.
- ELECTROLYTIC CELL: Electrolytic cell is an assembly of two electrodes in an electrolyte used for the electrolysis of a substance.
In an electrolytic cell, oxidation occurs at the anode (positive electrode) and reduction at the cathode (negative electrode).
EVALUATION
- Define the following terms
[a] electrolysis [b] electrode
- Explain the effect of electricity on matter
MECHANISM OF ELECTROLYSIS
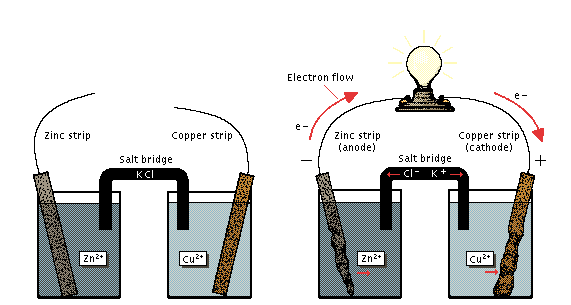
The electrolysis of a given electrolyte can be carried out in the electrolytic cell in two forms.
- Using the molten form of the electrolytes
ii Using the solution form of the electrolyte
- Using the molten (fused) form of the electrolyte, only two opposite ions from the electrolyte are present. Example, molten NaCl contains Na+ and Cl– ions only. Na+ ions migrate to the cathode to accept electrons and become discharged to produce neutral Na atoms.
Na+(s) + e– Na(s)
While chloride ions migrate to the anode to give up electrons and become discharged to produce Cl atoms which pair up to form chlorine gas, Cl2.
Cl– Cl + e–
Cl +Cl Cl2(g)
There is no competition for the discharge of ions at the electrodes.
- Using the solution form of the electrolysis, ions are produced from the electrolytes and from the solvent usually water, H2O. Two opposite ions from the electrolyte e.g Na+ and Cl– from NaCl and two from water, H+ and OH–. In such cases, the cations and anions of both the electrolyte and the solvent will migrate to the cathode and the anode respectively where they will compete with one another to be discharged. The products formed at the electrodes depend on which ions are preferentially discharged, the ions from the electrolytes or from the solvent.
PREFERENTIAL DISCHARGE OF IONS DURING ELECTROLYSIS
FACTORS AFFECTING PREFERENTIAL DISCHARGE OF IONS
The discharge of ions at the electrodes is governed by three conditions, namely
- The position of ions in the electrochemical series.
- The concentration of ions.
- The nature of the electrodes
RELATIVE POSITIONS OF THE IONS IN THE ELECTROCHEMICAL SERIES
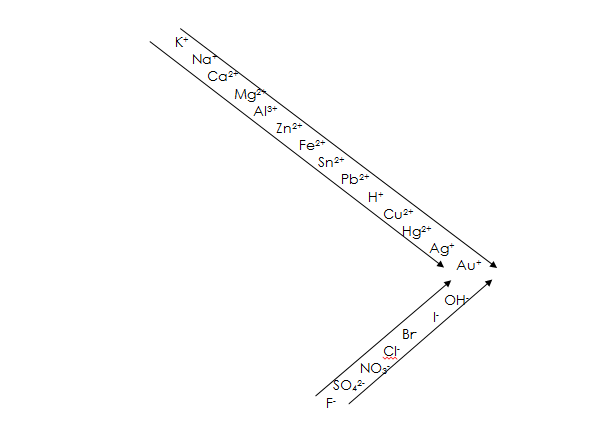
When all other factors are constant a cation (cathode ion) which is lowest in the series (less electropositive) will be discharged in preference to the one higher up (more electropositive). This is because the less electropositive element gains electron(s) more readily from the cathode and so become reduced and discharged as a neutral atom while the more electropositive element remains in the solution as positive ions.
NOTE: K+, Na+ and Ca+ are never discharge at all from aqueous solution. This is because of the large gap between them and H+. However, K+, Na+ and Ca+ are discharged during the electrolysis of their molten salt.
An anion which is higher up in the series (less electronegative) is preferentially discharged to the one lower down the series (more electronegative). This is because the less electronegative ion loses electron(s) more readily than the more electronegative ion.
NOTE: F–, SO42- and NO3– are never discharged from the aqueous solution because of the large gap between them and OH–.
CONCENTRATION OF IONS
When the concentration of an ion in the electrolyte is increased, the ion tends to increase its chances of being discharged. The influence of concentration, however, is effective only when the two competing ions are closely positioned in the electrochemical series. The effect of concentration becomes less important as the positions of the competing ions become further apart in the series.
NATURE OF ELECTRODES
Inert electrode (e.g Platinum and graphite) do not take part in the electrolytic reactions. However, platinum is attacked by liberated chlorine and graphite is attacked by liberated oxygen. Some electrodes have a strong affinity (love) for certain ion and influence the discharge of such ion. For example, in the electrolysis of aqueous NaCl using mercury cathode, Na+ will be discharged at the cathode to form sodium amalgam, Na/Hg
Na+(aq) + Hg(s) + e- Na/Hg(l)
Also, in the electrolysis of CuSO4 solution using copper anode, neither the SO42- nor the OH– will be discharged. Rather, the Cu atoms will lose electrons more readily and go into solution as Cu2+, hence, the copper anode if known as reactive electrode
GENERAL EVALUATION/REVISION
1 State the modern ionic theory.
- Name three strong and two weak electrolytes.
- Explain how the position of a named cation and anion determine the discharge of the ion during electrolysis
- State TWO differences between a conductor and an electrolyte
- Describe how you can separate a mixture of lead (II) chloride, sodium chloride and ammonium chloride
READING ASSIGNMENT
New School Chemistry for Senior Secondary Schools by O. Y. Ababio (6th edition) Pages 200-204
WEEKEND ASSIGNMENT
SECTION A: Write the correct option ONLY
- In an electrolytic cell, the chemical reaction which takes place at the anode is A. hydrolysis B. neutralization C. oxidation D. reduction
- Which of the following is a good conductor of electricity? A. Brine B. Ethanol C. Petrol D. Kerosene
- Which of the statements on electrolytes and conductors is/are true?
i. Electrolytes are compounds while conductors are elements
ii. Electrolytes contain mobile ions while conductors consist of mobile electrons
iii. Electrolytes can be solutions while conductors are usually solids
iv. Electrolytes and conductors can both be decomposed by an electric current
A. I, III and IV B. II, III and IV C. I, II and III D.I, III and IV
6. Which of the following accounts for the differences in the mode of conduction of electricity by metals and aqueous solutions?
- Electrons are present in metals but not in salt solutions.
- Metals are conductor while salts are electrolytes.
- Electricity is carried by mobile electrons in metals but by ions in an aqueous salt solution.
- Salts ionize in aqueous solution while metals do not.
7. Which of the following combination of ions is present in an aqueous solution of sodium chloride? A. H+ and Na+ only B. H+, Na+ and OH+ only C. OH– and Cl– only D. Na+, H+, Cl– and OH– only
SECTION B
- Define the following terms
- Electrolysis b. Electrolyte c. Electrode d. Electrolytic cell
- Draw a diagram of a simple electrolytic cell
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about Electrolysis of Specified Electrolytes. We are very much eager to meet you there.

THIS IS REALLY HELPFUL PEOPLE SHOULD GET TO KNOW ABOUT THIS.
This’s good enough
this is very beneficial to me💕