Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about Unsaturated Hydrocarbons (Alkenes). We hope you enjoy the class!

CONTENT
- Nomenclature
- Preparation, Properties and Uses.
UNSATURATED HYDROCARBONS
These are hydrocarbons in which carbon atoms join with each other by multiple bonds. The multiple bonds can be double bonds e.g. Alkenes or triple bonds e.g. Alkynes.
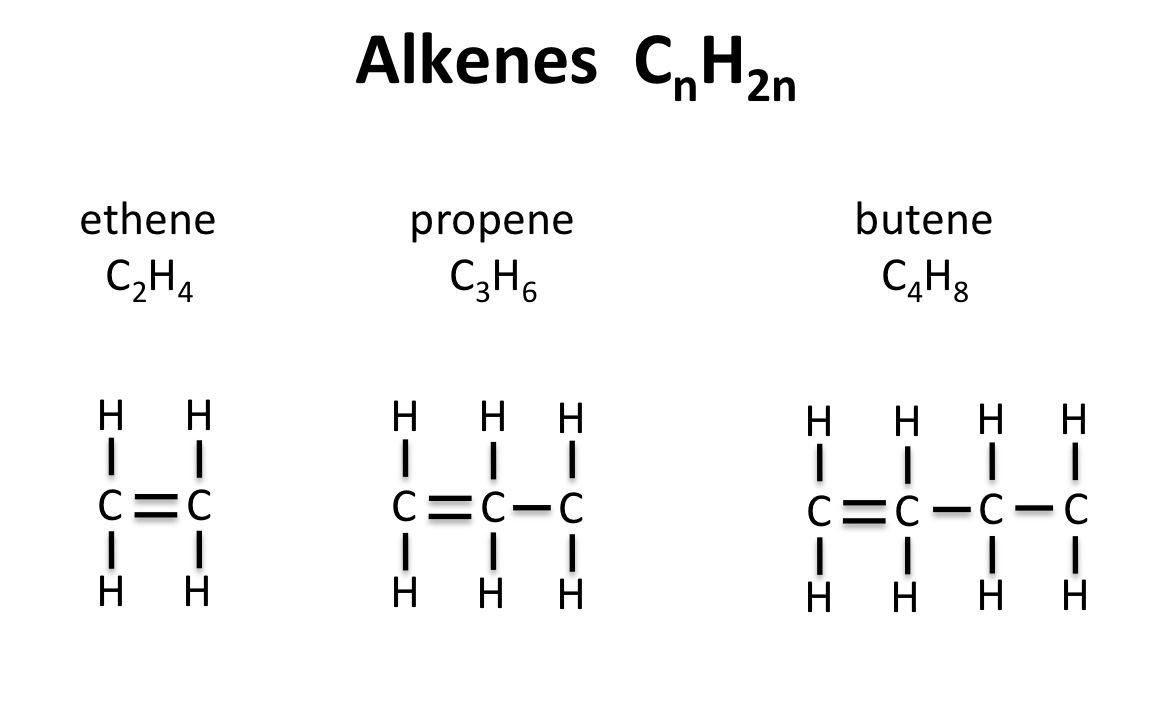
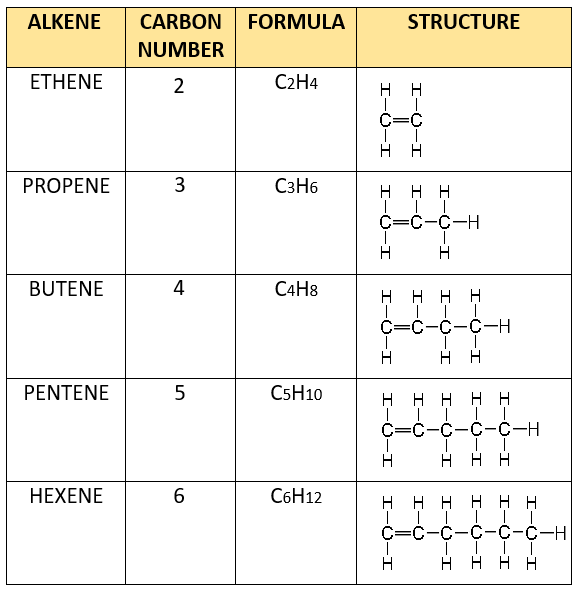
NOMENCLATURE
The process of naming in alkenes is obtained by substituting “ane” in alkane with ‘ene’ e.g. Ethane changes to Ethene, propane to propene
LABORATORY PREPARATION
Ethene is prepared by heating ethanol with excess concentrated tetraoxosulphate(VI) acid at 170o C. The acid acts as a dehydrating agent by removing water from the ethanol. Thus the process is called dehydration.
The reaction occurs in two stages
C2H5OH(aq) + H2SO4(aq) = C2H5HSO4(aq) + H2O(l)
C2H5HSO4(aq) = C2H4(g) + H2SO4.
The overall reaction is represented by the equation
C2H5OH(aq) H2SO4 = C2H4(g) + H2SO4(aq) -H2O
PHYSICAL PROPERTIES
- Ethene is a colourless gas with a faint sweetish smell.
- It is sparingly soluble in water.
- It is slightly less dense than air.
- It has no action on litmus paper.
Evaluation
- How would you prepare a jar of ethene gas in the laboratory?
- Mention four physical properties of Ethene.
CHEMICAL PROPERTIES
- Combustion: Ethene undergoes combustion in air or in the presence of oxygen and produces carbon (IV) oxide and steam.
C2H4(g) + 3O2(g) 2CO2(g) + 2H2O(l)
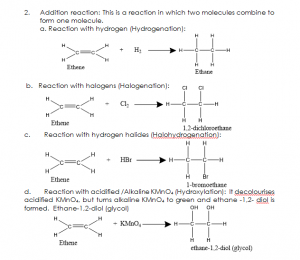
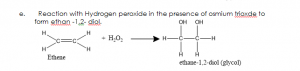
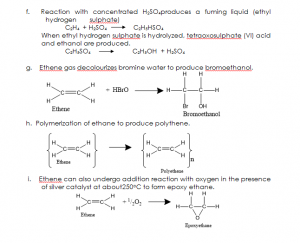
- Addition reaction: This is a reaction in which two molecules combine to form one molecule.
a. Reaction with hydrogen (Hydrogenation):
USES OF ETHENE: Ethene is used
- In the manufacture of plastics.
- In making synthetic rubber.
- To hasten the ripening of fruits.
- In the production of other organic compounds e.g halo-alkane, ethane and ethanol.
GENERAL Evaluation/REVISION
- Write balanced equations to show the reaction of ethene with the following:
- Bromine water
- Chlorine water
- Acidified KMnO4
- State four uses of ethene.
- Why is an empty flask inserted between the flat bottom flask and the conical flask holding the drying agent in the laboratory preparation of ethene?
- State THREE factors that determine the spontaneity of a chemical reaction.
- 92g of ethanol raised the temperature of 100g of water from 298K to 312.3K when burned completely. What is the heat of combustion of ethanol?
READING ASSIGNMENT
New School Chemistry for Senior Secondary School by O.Y. Ababio (6thedition) Pages 532-535
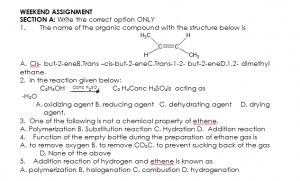
SECTION B
- Write and name the geometric isomers of the compound with the molecular formula C5H10
- Write a balanced chemical equation to show how ethene reacts with the following:
a. concentrated H2SO4 b. bromine water c. acidified KMnO4
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about Unsaturated Hydrocarbons (Alkynes). We are very much eager to meet you there.
